Nadph tetrasodium salt
Synonym(s):β-NADPH;Dihydronicotinamide adenine dinucleotide phosphate tetrasodium salt;NADPH Na4;TPNH2 Na4;Triphosphopyridine nucleotide reduced tetrasodium salt
- CAS NO.:2646-71-1
- Empirical Formula: C21H31N7NaO17P3
- Molecular Weight: 769.42
- MDL number: MFCD00036263
- EINECS: 220-163-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 08:49:36
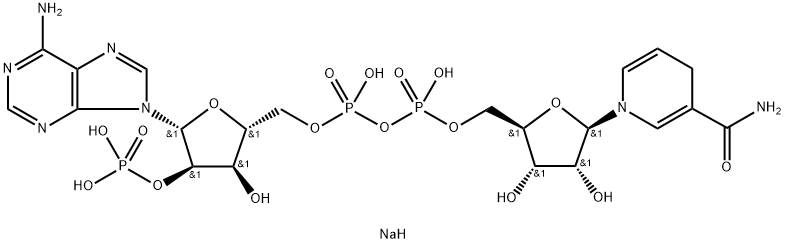
What is Nadph tetrasodium salt?
Description
NADPH is the reduced form of the coenzyme NADP+; used in anabolic reactions such as lipid and nucleic acid synthesis, which require NADPH as a reducing agent.
NADPH, Tetrasodium Salt is a A ubiquitous coenzyme that acts as an electron donor in many reactions utilizing dehydrogenase and reductase enzymes. It is generated by reduction of the electron acceptor NADP+. The following biological pathways involve NADPH: formation of carbohydrate from CO2 during photosynthesis, maintenance of high levels of reduced glutathione in erythrocytes, reduction of thioredoxin.
Chemical properties
off-white to light yellow powder
The Uses of Nadph tetrasodium salt
One of the biologically active forms of nicotinic acid. Differs from NAD by an additional phosphate group at the 2’position of the adenosine moiety. Serves as a coenzyme of hydrogenases and dehydroge nases. Present in living cells primarily in the reduced form (NADPH) and is involved in synthetic reactions.
The Uses of Nadph tetrasodium salt
NADPH tetra sodium salt is used as an ubiquitous cofactor and biological reducing agent. β-NADPH is a coenzyme found in all living cells and is involved in redox reactions carrying electrons from one reaction to another. It is used as an electron donor, cofactor for many redox enzymes including nitric oxide synthetase.
The Uses of Nadph tetrasodium salt
β-Nicotinamide adenine dinucleotide 2′-phosphate (NADP+) and β-Nicotinamide adenine dinucleotide 2′-phosphate, reduced (NADPH) comprise a coenzyme redox pair (NADP+:NADPH) involved in a wide range of enzyme catalyzed oxidation reduction reactions. The NADP+/NADPH redox pair facilitates electron transfer in anabolic reactions such as lipid and cholesterol biosynthesis and fatty acyl chain elongation. The NADP+/NADPH redox pair is used in a variety of antioxidation mechanism where it protects agains reactive oxidation species accumulation. NADPH is generated in vivio by the pentose phosphate pathway (PPP).
What are the applications of Application
NADPH tetrasodium salt is a ubiquitous coenzyme that acts as an electron donor in many reactions utilizing dehydrogenase and reductase enzymes.
What are the applications of Application
NADPH is the reduced form of the electron acceptor nicotinamide adenine dinucleotide phosphate (NADP+) and acts as an electron donor in various biological reactions. In plants, NADPH is produced by ferredoxin-NADP+ reductase in the last step of the electron chain during photosynthesis. In animals is it predominantly produced by the pentose phosphate pathway, but is also generated by key mitochondrial enzymes. NADPH provides the reducing equivalents for biosynthetic reactions and the oxidation-reduction involved in protecting against the toxicity of reactive oxygen species.It is also used for the synthesis of lipids and cholesterol and during the process of fatty acid chain elongation.
General Description
A ubiquitous coenzyme that acts as an electron donor in many reactions utilizing dehydrogenase and reductase enzymes. It is generated by reduction of the electron acceptor NADP+. The following biological pathways involve NADPH: formation of carbohydrate from CO2 during photosynthesis, maintenance of high levels of reduced glutathione in erythrocytes, reduction of thioredoxin.
Biological Activity
NADPH is an electron donor and a cofactor for many redox enzymes including nitric oxide synthetase.
β-Nicotinamide adenine dinucleotide 2′-phosphate (NADP+) and β-Nicotinamide adenine dinucleotide 2′-phosphate, reduced (NADPH) comprise a coenzyme redox pair (NADP+:NADPH) involved in a wide range of enzyme catalyzed oxidation reduction reactions. The NADP+/NADPH redox pair facilitates electron transfer in anabolic reactions such as lipid and cholesterol biosynthesis and fatty acyl chain elongation. The NADP+/NADPH redox pair is used in a variety of antioxidation mechanism where it protects agains reactive oxidation species accumulation. NADPH is generated in vivio by the pentose phosphate pathway (PPP).
Biochem/physiol Actions
NADPH is an electron donor and a cofactor for many redox enzymes including nitric oxide synthetase.
Storage
-20°C
Purification Methods
Purification is mostly similar to that of NADH above. [Beilstein 26 III/IV 3671.]
Properties of Nadph tetrasodium salt
| Melting point: | >250°C (dec.) |
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| solubility | 10 mM NaOH: soluble50mg/mL, clear |
| form | Powder |
| color | White to Off-white |
| Water Solubility | Soluble in water (50 mg/ml). |
| Sensitive | Light Sensitive |
| Merck | 14,6348 |
Safety information for Nadph tetrasodium salt
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Nadph tetrasodium salt
| InChIKey | ZQJHSLJFGRIJQN-LRANKJFRNA-N |
Nadph tetrasodium salt manufacturer
Spectrochem Pvt., Ltd.
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Dihydronicotinamide-adenine dinucleotide phosphate tetrasodium salt 98%View Details
Dihydronicotinamide-adenine dinucleotide phosphate tetrasodium salt 98%View Details -
 β-Nicotinamide adenine dinucleotide 2′-phosphate reduced tetrasodium salt hydrate ≥95% (HPLC) CAS 2646-71-1View Details
β-Nicotinamide adenine dinucleotide 2′-phosphate reduced tetrasodium salt hydrate ≥95% (HPLC) CAS 2646-71-1View Details
2646-71-1 -
![β-Nicotinamide Adenine Dinucleotide Phosphate Tetrasodium Salt reduced form [for Biochemical Research] CAS 2646-71-1](https://img.chemicalbook.in//Content/image/CP5.jpg) β-Nicotinamide Adenine Dinucleotide Phosphate Tetrasodium Salt reduced form [for Biochemical Research] CAS 2646-71-1View Details
β-Nicotinamide Adenine Dinucleotide Phosphate Tetrasodium Salt reduced form [for Biochemical Research] CAS 2646-71-1View Details
2646-71-1 -
 ß-Nicotinamide Adenine Dinucleotide Phosphate Tetrasodium Salt (Reduced) (ß-NADPH.Na4) for MB CAS 2646-71-1View Details
ß-Nicotinamide Adenine Dinucleotide Phosphate Tetrasodium Salt (Reduced) (ß-NADPH.Na4) for MB CAS 2646-71-1View Details
2646-71-1 -
 Beta-nicotinamide adenine dinucleotide phosphate (reduced form CAS 2646-71-1View Details
Beta-nicotinamide adenine dinucleotide phosphate (reduced form CAS 2646-71-1View Details
2646-71-1 -
 NADPH tetrasodium salt, 98% CAS 2646-71-1View Details
NADPH tetrasodium salt, 98% CAS 2646-71-1View Details
2646-71-1 -
 Beta-nicotinamide adenine dinucleotide phosphate tetrasodium salt (reduced form) 95% CAS 2646-71-1View Details
Beta-nicotinamide adenine dinucleotide phosphate tetrasodium salt (reduced form) 95% CAS 2646-71-1View Details
2646-71-1 -
 β-Nicotinamide adenine dinucleotide 2′-phosphate reduced tetrasodium salt hydrate CAS 2646-71-1View Details
β-Nicotinamide adenine dinucleotide 2′-phosphate reduced tetrasodium salt hydrate CAS 2646-71-1View Details
2646-71-1
