β-Nicotinamide adenine dinucleotide
Synonym(s):NAD;DPN;β-NAD;Nadide;β-Nicotinamide adenine dinucleotide hydrate
- CAS NO.:53-84-9
- Empirical Formula: C21H27N7O14P2
- Molecular Weight: 663.43
- MDL number: MFCD02688274
- EINECS: 200-184-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-25 20:05:57
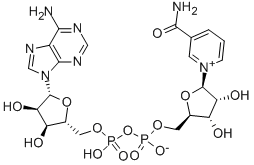
What is β-Nicotinamide adenine dinucleotide?
Description
β-Nicotinamide adenine dinucleotide (NAD+) plays a major role in metabolism as a cofactor and mobile electron acceptor. NAD+ is a required oxidizing cosubstrate for many enzymes. It is reduced to NADH (Cat# N-035) which carries electrons to the electron transport chain for subsequent oxidative phorphorylation and ATP production. NAD+ is capable of donating ADP-ribose moieties to a protein, producing nicotinamide in the process. Sirtuin enzymes use NAD+ as a substrate to deacetylate proteins and direct activity between the nucleus and mitochondria. NAD+ is regenerated by fermentation and by oxidative phosphorylation.
Chemical properties
Beta-Nicotinamide adenine dinucleotide is a hygroscopic white powder. It should be stored desiccated. Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Recommended storage temperature -20°C.
History
The coenzyme NAD+ was first discovered by British biochemists Arthur Harden and William John Young in 1906. They observed that the addition of boiled and filtered yeast extract significantly increased the rate of alcoholic fermentation in unboiled yeast extracts. They named the unidentified factor responsible for this effect a coferment. By extensively purifying it from yeast extracts, Hans von Euler-Chelpin identified this heat-stable factor as a nucleotide sugar phosphate. In 1936, German scientist Otto Heinrich Warburg demonstrated the role of this nucleotide coenzyme in hydride transfer and determined the nicotinamide portion as the site of redox reactions. Vitamin precursors of NAD+ were first identified in 1938, when Conrad Elvehjem showed that liver has an "anti-black tongue" activity in the form of nicotinamide. Then, in 1939, he provided the first strong evidence that niacin is used to synthesize NAD+.
The Uses of β-Nicotinamide adenine dinucleotide
β-Nicotinamide Adenine Dinucleotide is a coeznyme consisting of an adenine base and a nicotinamide base connected by a pair of bridging phosphate group. β-Nicotinamide Adenine Dinucleotide acts as a c oenzyme in redox reactions, as a donor of ADP-ribose moieties in ADP-ribosylation reactions and also as a precursor of the second messenger molecule cyclic ADP-ribose. β-Nicotinamide Adenine Dinucleot ide also acts as a substrate for bacterial DNA ligases and a group of enzymes called sirtuins that use NAD+ to remove acetyl groups from proteins.
What are the applications of Application
NAD+, Free Acid is a signaling molecule, enzyme cofactor, oxidizing agent, and major electron acceptor.
Definition
ChEBI: β-Nicotinamide adenine dinucleotide (NAD) is the oxidized form of β-Nicotinamide Adenine Dinucleotide. It exists as an anion under normal physio-logic conditions. It is functionally related to a deamido-NAD zwitterion. It is a conjugate base of a NAD(+). It is found widely in nature and is involved in numerous enzymatic reactions in which it serves as an electron carrier by being alternately oxidized (NAD+) and reduced (NADH). (Dorland, 27th ed)
What are the applications of Application
β-Nicotinamide adenine dinucleotide (β-NAD) is a cofactor of alcohol dehydrogenase and acts as a neuromodulator and an inhibitory neurotransmitter in visceral smooth muscles. The NAD/NADH ratio has a role in the regulation of intracellular redox potential. It thereby influences metabolic reactions in vivo. It has been used for the preparation of deacetylated tubulin. It has also been used for UDP-glucose-6-hydrogenase (UGDH) enzyme activity assay of orital fibroblast cell lysates.
β-Nicotinamide adenine dinucleotide (NAD+) and β-Nicotinamide adenine dinucleotide, reduced (NADH) comprise a coenzyme redox pair (NAD+:NADH) involved in a wide range of enzyme catalyzed oxidation reduction reactions. In addition to its redox function, NAD+/NADH is a donor of ADP-ribose units in ADP-ribosylaton (ADP-ribosyltransferases; poly(ADP-ribose) polymerases ) reactions and a precursor of cyclic ADP-ribose (ADP-ribosyl cyclases).
Benefits
The benefits of NAD+Therapy directly related to mental health treatment include:
Restores cellular energy, metabolism and promotes cellular repair
Helps regulate cellular defense and reduces inflammation
Elevates mood and generates the feeling of calmness and happiness
Supports healthy aging and cognitive function
NAD infusion therapy is most effective when multiple infusions are conducted regularly over time. Long-term maintenance and cellular support contribute to less overall damage to your mitochondria, a slower aging rate, and better cognitive health.
General Description
β-Nicotinamide adenine dinucleotide (NAD) is a ubiquitously found electron carrier and a cofactor. NAD+ contains an adenylic acid and a nicotinamide-5′-ribonucleotide group linked together by a pyrophosphate moiety. In NAD+ complexes, the enzyme-cofactor interactions are highly conserved.
Biological Activity
NAD+, known more formally as nicotinamide adenine dinucleotide, is a signaling molecule as well as a cofactor or substrate for many enzymes. It acts as an oxidizing agent, accepting electrons from other molecules while being converted to its reduced form, NADH. NAD+ is also essential for the activity of several enzymes, including poly(ADP)-ribose polymerases and cADP-ribose synthases. For example, it is used by some sirtuins to mediate protein deacetylation, producing O-acetyl-ADP-ribose and nicotinamide as well as the deacetylated protein.
Biochem/physiol Actions
β-Nicotinamide adenine dinucleotide (β-NAD) is an electron carrier and a cofactor, significantly involved in enzyme-catalyzed oxido-reduction processes and many genetic processes. NAD cycles between the oxidized (NAD+) and reduced (NADH) forms to maintain a redox balance necessary for continued cell growth. NAD is also involved in microbial catabolism. β-NAD acts as a substrate for various enzymes in several cellular processes.
Side Effects
In the studies conducted thus far, people taking between 1000mg-2000mg per day have reported zero long term side effects. However, during the active infusion, some people may feel temporary nausea or stomach discomfort.
Purification Methods
NAD is purified by paper chromatography or better on a Dowex-1 ion-exchange resin. The column is prepared by washing with 3M HCl until free of material absorbing at 260nm, then with water, 2M sodium formate until free of chloride ions and, finally, with water. NAD, as a 0.2% solution in water, is adjusted with NaOH to pH 8, and adsorbed onto the column, washed with water, and eluted with 0.1M formic acid. Fractions with strong absorption at 360nm are combined, acidified to pH 2.0 with 2M HCl, and cold acetone (ca 5L/g of NAD) is added slowly and with constant agitation. It is left overnight in the cold, then the precipitate is collected in a centrifuge, washed with pure acetone and dried under vacuum over CaCl2 and paraffin wax shavings [Kornberg Methods Enzymol 3 876 1957]. It has been purified by anion-exchange chromatography [Dalziel & Dickinson Biochemical Preparations 11 84 1966.] The purity is checked by reduction to NADH (with EtOH and yeast alcohol dehydrogenase) which has 340mn 6220 M-1cm-1. [Todd et al. J Chem Soc 3727, 3733 1957.] [pKa, Lamborg et al. J Biol Chem 231 685 1958.] The free acid crystallises from aqueous Me2CO with 3H2O and has m 140-142o. It is stable in cold neutral aqueous solutions in a desiccator (CaCl2) at 25o, but decomposes at strong acid and alkaline pH. Its purity is checked by reduction with yeast alcohol dehydrogenase and EtOH to NADH and noting the OD at 340nm. Pure NADH (see below) has 340 6.2 x 104 M-1cm-1, i.e. 0.1mole of NADH in 3mL and in a 1cm path length cell has an OD at 340nm of 0.207. [Beilstein 26 IV 3644.]
References
1. Pollak N.; D?lle C.; Ziegler M. The power to reduce: pyridine nucleotides - small molecules with a multitude of functions. Biochem. J. 2007, 402(2), 205-18. DOI:10.1042/BJ20061638
2. Unden G, Bongaerts J. Alternative respiratory pathways of Escherichia coli: energetics and transcriptional regulation in response to electron acceptors. Biochim. Biophys. Acta. 1997, 1320(3), 217-34. DOI:10.1016/S0005-2728(97)00034-0
3. Houtkooper, R.H., Cantó, C., Wanders, R.J., et al. The secret life of NAD+: An old metabolite controlling new metabolic signaling pathways. Endocr. Rev. 31(2), 194-223 (2010). DOI:10.1210/er.2009-0026
4. Experimental and theoretical electron density studies in large molecules: NAD+, beta-nicotinamide adenine dinucleotide DOI:10.1021/JP034478B
5. A Mechanism of Adsorption of beta-Nicotinamide Adenine Dinucleotide on Graphene Sheets: Experiment and Theory DOI:10.1002/chem.200900399
6. β-Nicotinamide Adenine Dinucleotide (β-NAD) Inhibits ATP-Dependent IL-1β Release from Human Monocytic Cells DOI:10.3390/ijms19041126
7. Oxidation of β-Nicotinamide Adenine Dinucleotide (NADH) by Au Cluster and Nanoparticle Catalysts Aiming for Coenzyme Regeneration in Enzymatic Glucose Oxidation DOI:10.1021/acssuschemeng.0c01893
Properties of β-Nicotinamide adenine dinucleotide
| Melting point: | 140-142 °C (decomp) |
| alpha | D20 -31.5° (c = 1.2 in water) |
| storage temp. | -20°C |
| solubility | H2O: 50 mg/mL |
| form | Powder |
| color | White |
| Odor | Odorless |
| PH | ~3.0 (50mg/mL in water) |
| Water Solubility | Soluble in water at 50mg/ml |
| Merck | 14,6344 |
| BRN | 3584133 |
| Stability: | Stable. Hygroscopic. Incompatible with strong oxidizing agents. |
| EPA Substance Registry System | Adenosine 5'-(trihydrogen diphosphate), P'.fwdarw.5'-ester with 3-(aminocarbonyl)-1-.beta.-D-ribofuranosylpyridinium, inner salt (53-84-9) |
Safety information for β-Nicotinamide adenine dinucleotide
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H371:Specific target organ toxicity, single exposure |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for β-Nicotinamide adenine dinucleotide
| InChIKey | BAWFJGJZGIEFAR-WWRWIPRPSA-N |
Abamectin manufacturer
Pallav Chemicals And Solvents Pvt Ltd
New Products
4-Fluorophenylacetic acid (S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid 5-Aminoimidazole-4-Carbonitrile 4-chloro-3,5-dinitropyridine 2'-Methoxy-biphenyl-2-carboxaldehyde 2-(2-Aminoethyl)isothiourea dihydrobromide, 1-(4-chlorophenyl)propan-1-one 2-Ethyl-4-methyl-1-pentanol DIISOPROPYL MALONATE PENTAFLUOROPHENOL 2-Aminonicotinic acid 6-(4-AMINOPHENYL)-5-METHYL-4,5-DIHYDRO-3(2 H)-PYRDAZINONE β-BUTYROLACTONE 3-OXO-CYCLOBUTANECARBOXYLIC ACID 3-methyl xanthine 1H-Pyrazole-3-carboxylic acid [1,1'-Biphenyl]-4-carboxylic acid (3aR,4R,5R,6aS)-hexahydro-2-oxo-4-[(1E)-3-oxo-4-[3- (trifluoromethyl)phenoxy]-1-buten-1-yl]-2H-cyclopenta[b]furan-5-yl ester 2H-Cyclopenta[b]furan-2,5-diol, hexahydro-4-[(1E,3R)-3-hydroxy-4-[3-(trifluoromethyl)phenoxy]-1- buten-1-yl]-, (3aR,4R,5R,6aS)- 2,5-Dibromopyridine Dimethyl (2-oxo-4-phenylbutyl)phosphonate S-(2-Chloro-3-nitrophenyl) O-ethyl carbonodithioate 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carboxylic acid 2,4-Dichloro-1-[2-nitro-4-(trifluoromethyl)phenoxy]benzeneRelated products of tetrahydrofuran

You may like
-
 53-84-9 Nadide 98%View Details
53-84-9 Nadide 98%View Details
53-84-9 -
 Nicotimide Adenine Dinucleotide 95% (D, DPN) 99%View Details
Nicotimide Adenine Dinucleotide 95% (D, DPN) 99%View Details
53-84-9 -
 β-NAD (NAD, DPN) CAS 53-84-9View Details
β-NAD (NAD, DPN) CAS 53-84-9View Details
53-84-9 -
![β-Nicotinamide Adenine Dinucleotide oxidized form [for Biochemical Research] CAS 53-84-9](https://img.chemicalbook.in//Content/image/CP5.jpg) β-Nicotinamide Adenine Dinucleotide oxidized form [for Biochemical Research] CAS 53-84-9View Details
β-Nicotinamide Adenine Dinucleotide oxidized form [for Biochemical Research] CAS 53-84-9View Details
53-84-9 -
 Nicotinamide adenine dinucleotide free acid CAS 53-84-9View Details
Nicotinamide adenine dinucleotide free acid CAS 53-84-9View Details
53-84-9 -
 NICOTINAMIDE ADENINE DINUCLEOTIDE For Biochemistry CAS 53-84-9View Details
NICOTINAMIDE ADENINE DINUCLEOTIDE For Biochemistry CAS 53-84-9View Details
53-84-9 -
 (3R,4R)-1-benzyl-N,4- dimethylpiperidin-3-amine 477600-70-7 99%View Details
(3R,4R)-1-benzyl-N,4- dimethylpiperidin-3-amine 477600-70-7 99%View Details
477600-70-7 -
 Ortho Phenylene Diamine (OPDA) 95-54-5 99%View Details
Ortho Phenylene Diamine (OPDA) 95-54-5 99%View Details
95-54-5