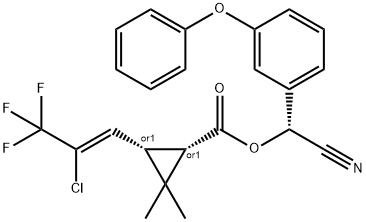
Trifloxystrobin
Synonym(s):Methyl (E)-α-methoxyimino-2-[(E)-1-(3-trifluoromethylphenyl)ethylidenaminooxymethyl]phenylacetate
- CAS NO.:141517-21-7
- Empirical Formula: C20H19F3N2O4
- Molecular Weight: 408.37
- MDL number: MFCD09953763
- EINECS: 480-070-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04
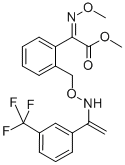
What is Trifloxystrobin?
Chemical properties
Off-White to Pale Beige Solid
The Uses of Trifloxystrobin
Trifloxystrobin is a broad-spectrum foliar fungicide used in plant pritection. Trifloxystrobin functions by inhibiting fungal spore germination.
The Uses of Trifloxystrobin
Agricultural fungicide.
What are the applications of Application
Trifloxystrobin is a strobilurin class fungicide
Definition
ChEBI: Trifloxystrobin is the methyl ester of (2E)-(methoxyimino)[2-({[(E)-{1-[3-(trifluoromethyl)phenyl]ethylidene}amino]oxy}methyl)phenyl]acetic acid. A foliar applied fungicide for cereals which is particularly active against Ascomycetes, Deuteromycetes and Oomycetes It has a role as a mitochondrial cytochrome-bc1 complex inhibitor and an antifungal agrochemical. It is an oxime O-ether, an organofluorine compound, a methyl ester and a methoxyiminoacetate strobilurin antifungal agent.
Metabolism
Trifloxystrobin is described as “mesostemic” due to its ability to redistribute to untreated plant parts through vapor action, limited but effective cuticular penetration, and translaminarmovement by diffusion (30). It is rainfast by virtue of its high affinity for the waxy cuticular layer.
Toxicity evaluation
Trifloxystrobin has an acute oral LD50 > 5,000 and an acute dermal LD50 > 2,000 mg/kg in rats. It is not a skin or eye irritant, is nonmutagenic and nonteratogenic. It shows rapid absorption and elimination in the rat. It has no toxicity to birds in acute studies (LD50 > 2,000 mg/kg) but has an LC50 of 0.015 mg/L in rainbow trout. The bee LD50 = 200 μg/bee. Environmental fate studies show it to be hydrolytically stable at pH 5, with a DT50 of 11.4 weeks at pH 7. It has a photolytic DT50 of 31.5 h at pH 7 (25 ?C) in water, a soil adsorption coefficient (Koc) of 1,642-3,745 ml/g, and a soil DT50 of 5.4 days under field conditions.
Properties of Trifloxystrobin
| Melting point: | 72.9° |
| Boiling point: | bp~312° |
| Density | 1.21±0.1 g/cm3(Predicted) |
| Flash point: | >70 °C |
| storage temp. | Sealed in dry,2-8°C |
| solubility | Chloroform: Slightly Soluble; Methanol: Slightly Soluble |
| form | neat |
| form | Solid |
| color | White to off-white |
| Stability: | Hygroscopic, Moisture Sensitive |
| EPA Substance Registry System | Trifloxystrobin (141517-21-7) |
Safety information for Trifloxystrobin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H332:Acute toxicity,inhalation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for Trifloxystrobin
| InChIKey | MGNJFZURPQVGJE-BWAHOGKJSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 Trifloxystrobin CAS 141517-21-7View Details
Trifloxystrobin CAS 141517-21-7View Details
141517-21-7 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
