Triethylenethiophosphoramide
Synonym(s):Thio-TEPA;Tiofosfamid;Tiofosyl;Tiofozil;Tio-tef
- CAS NO.:52-24-4
- Empirical Formula: C6H12N3PS
- Molecular Weight: 189.22
- MDL number: MFCD00145452
- EINECS: 200-135-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
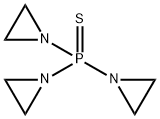
What is Triethylenethiophosphoramide?
Description
Thiotepa, a tertiary aziridine, is less reactive than quaternary aziridinium compounds and is classified as a weak alkylator. It is possible for the nitrogen atoms to be protonate before reacting with DNA (a positively charged aziridine is more reactive than the un-ionized aziridine), but the electron-withdrawing effect of the sulfur atom decreases the pKa to approximately six, which keeps the percentage ionized at pH 7.4 relatively low. Thiotepa undergoes oxidative desulfuration, forming an active cytotoxic metabolite known as TEPA (triethylenephosphoramide).
Chemical properties
white crystals or powder
Chemical properties
Thiotepa is a crystalline substance.
Originator
Thio-Tepa,Lederle,US,1959
The Uses of Triethylenethiophosphoramide
Tri(1-aziridinyl)phosphine sulfide is useful for the treatment of cancers, especially cancers resistant to chemotherapy. Antineoplastic. Thio-TEPA (N,N?N?-triethylenethiophosphoramide) is used as a cancer chemotherapeutic, alkylating agent. It is used to treat various kinds of cancer such as breast, ovarian and bladder cancer. It is also used as conditioning treatment prior to hematopoietic progenitor cell transplantation (HPCT)
The Uses of Triethylenethiophosphoramide
This substance is listed as a known human carcinogen. It is useful for the treatment of cancers, especially cancers resistant to chemotherapy. Antineoplastic.
The Uses of Triethylenethiophosphoramide
suzuki reaction
The Uses of Triethylenethiophosphoramide
antiseborrheic, antipruritic
The Uses of Triethylenethiophosphoramide
Insect sterilant.
What are the applications of Application
Thio-TEPA is an alkylating agent
Indications
ThioTEPA is used a as conditioning treatment prior to allogeneic or autologous haematopoietic progenitor cell transplantation (HPCT) in haematological diseases in adult and paediatric patients. Also, when high dose chemotherapy with HPCT support it is appropriate for the treatment of solid tumours in adult and paediatric patients.
Background
N,N'N'-triethylenethiophosphoramide (ThioTEPA) is a cancer chemotherapeutic member of the alkylating agent group, now in use for over 50 years. It is a stable derivative of N,N',N''- triethylenephosphoramide (TEPA). It is mostly used to treat breast cancer, ovarian cancer and bladder cancer. It is also used as conditioning for Bone marrow transplantation. Its main toxicity is myelosuppression.
Indications
Although thiotepa is chemically less reactive than the nitrogen
mustards, it is thought to act by similar mechanisms.
Its oral absorption is erratic. After intravenous injection,
the plasma half-life is less than 2 hours. Urinary
excretion accounts for 60 to 80% of eliminated drug.
Thiotepa has antitumor activity against ovarian and
breast cancers and lymphomas. However, it has been
largely supplanted by cyclophosphamide and other nitrogen
mustards for treatment of these diseases. It is
used by direct instillation into the bladder for multifocal
local bladder carcinoma.
Nausea and myelosuppression are the major toxicities
of thiotepa. It is not a local vesicant and has been
safely injected intramuscularly and even intrathecally.
Definition
ChEBI: Thiotepa is a member of aziridines.
Manufacturing Process
A solution of 30.3 parts of triethylamine and 12.9 parts of ethylenimine in 180 parts of dry benzene is treated with a solution of 16.9 parts of thiophosphoryl chloride in 90 parts of dry benzene at 5°C to 10°C. Triethylamine hydrochloride is filtered off. The benzene solvent is distilled from the filtrate under reduced pressure and the resulting crude product is recrystallized from petroleum ether. The N,N',N''-triethylenethiophosphoramide had a melting point of 51.5°C.
Therapeutic Function
Antineoplastic
General Description
Odorless white crystalline solid.
General Description
The early success of the nitrogen mustards led researchers toinvestigate other compounds that contained a preformed aziridinering, and thiotepa resulted from this work. Thiotepa containingthe thiophosphoramide functionality was found to bemore stable than the oxa-analog (TEPA) but is metabolicallyconverted to TEPA by desulfuration in vivo.Thiotepa incorporatesa less reactive aziridine ring compared with thatformed in mechlorethamine. The adjacent thiophosphoryl iselectron withdrawing and, therefore, reduces the reactivity ofthe aziridine ring system. Although thiotepa is less reactivethan many other alkylating agents, it has been shown to formcross-links.
Air & Water Reactions
Water soluble.
Reactivity Profile
Triethylenethiophosphoramide polymerizes readily upon exposure to heat or moisture, especially at acidic pH.
Hazard
Confirmed carcinogen.
Fire Hazard
Flash point data for Triethylenethiophosphoramide are not available. Triethylenethiophosphoramide is probably combustible.
Biochem/physiol Actions
The unstable nitrogen-carbon groups alkylate with DNA which causes irreversible DNA damage. They stop tumor growth by crosslinking guanine nucleobases in DNA double-helix strands, directly attacking DNA. The DNA strands are unable to uncoil and separate which halts cell division.
Mechanism of action
Thiotepa and the TEPA metabolite readily enter the CNS after systemic administration, leading to dizziness, blurred vision, and headaches. More critically, these agents also are severe myelosuppressants and can induce leukopenia, thrombocytopenia, and anemia. Patients treated with thiotepa are at high risk for infection and hemorrhage.
Pharmacokinetics
The unstable nitrogen-carbon groups alkylate with DNA causing irrepairable DNA damage. They stop tumor growth by crosslinking guanine nucleobases in DNA double-helix strands, directly attacking DNA. This makes the strands unable to uncoil and separate. As this is necessary in DNA replication, the cells can no longer divide. These drugs act nonspecifically.
Clinical Use
This antineoplastic agent is most commonly employed in the treatment of ovarian and breast cancers, as well as papillary carcinoma of the bladder.
Side Effects
Patients have died from myelosuppression after intravesically administered thiotepa. The drug also causes damage to the hepatic and renal systems. Dose and/or administration frequency should be increased slowly, even if the initial response to the drug is sluggish, or unacceptable toxicity may result.
Safety Profile
Confirmed human carcinogen producing leukemia. Poison by ingestion, intraperitoneal, intravenous, and subcutaneous routes. Experimental teratogenic data. Human systemic effects by parenteral route: paresthesia, bone marrow changes, and leukemia. Experimental reproductive effects. Human mutation data reported. When heated to decomposition it emits very toxic fumes of POx, SOx, and NOx.
Synthesis
Thiotepa, tris(1-aziridinyl)phosphine sulfate (30.2.2.1), is made by reacting ethylenimine with phosphorous sulfochloride .
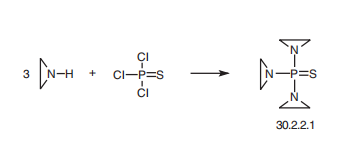
Potential Exposure
Used in the treatment of cancers resistant to chemotherapy. Antineoplastic: thiotepa has been prescribed for a wide variety of neoplastic diseases: adenocarcinomas of the breast and the ovary; superficial carcinoma of the urinary bladder; controlling intracavitary or localized neoplastic disease; lymphomas, such aslymphosarcomas and Hodgkin’s disease; as well as bronchogenic carcinoma.
Drug interactions
Potentially hazardous interactions with other drugs
Antipsychotics: avoid concomitant use with clozapine.
Avoid concomitant use with other myelosuppressive agents. Administration
Carcinogenicity
Thiotepa is known to be a human carcinogen based on sufficient evidence from studies in humans. Thiotepa was first listed in the Second Annual Report on Carcinogens in 1981 as reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals and insufficient evidenceof carcinogenicity from studies in humans. Thiotepa was reclassified as known to be a human carcinogen in the Eighth Report on Carcinogens in 1998.
Metabolism
Not Available
Metabolism
Thiotepa is extensively metabolised to triethylenephosphoramide (TEPA), the primary metabolite, and some of the other metabolites have cytotoxic activity and are eliminated more slowly than the parent compound. It is excreted in the urine: less than 2
% of a dose is reported to be present as unchanged drug or its primary metabolite.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. UN3249 Medicine, solid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Toxicity evaluation
One of the principal bond disruptions is initiated by alkylation of guanine at the N-7 position, which severs the linkage between the purine base and the sugar and liberates alkylated guanines. This causes DNA cross-linking and prevents the replication of rapidly dividing cells.
Incompatibilities
Tris(aziridinyl)phosphine sulfide polymerizes readily upon exposure to heat or moisture, especially at acidic pH. Incompatible with strong oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
Waste Disposal
It is inappropriate and possibly dangerous to the environment to dispose of expired or waste pharmaceuticals by flushing them down the toilet or discarding them to the trash. Household quantities of expired or waste pharmaceuticals may be mixed with wet cat litter or coffee grounds, double-bagged in plastic, discard in trash. Larger quantities shall carefully take into consideration applicable DEA, EPA, and FDA regulations. If possible return the pharmaceutical to the manufacturer for proper disposal being careful to properly label and securely package the material. Alternatively, the waste pharmaceutical shall be labeled, securely packaged and transported by a state licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or incinerator.
Properties of Triethylenethiophosphoramide
| Melting point: | 54-57 °C |
| Boiling point: | 270.2±23.0 °C(Predicted) |
| Density | 1.50±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | Soluble in benzene, acetone and methanol. |
| pka | 2.74±0.20(Predicted) |
| form | solid |
| color | white |
| Water Solubility | 19 g/100 mL (25 ºC) |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 52-24-4 |
| IARC | 1 (Vol. Sup 7, 50, 100A) 2012 |
| NIST Chemistry Reference | Thiotepa(52-24-4) |
| EPA Substance Registry System | Thiotepa (52-24-4) |
Safety information for Triethylenethiophosphoramide
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H300:Acute toxicity,oral H350:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Triethylenethiophosphoramide
| InChIKey | FOCVUCIESVLUNU-UHFFFAOYSA-N |
Triethylenethiophosphoramide manufacturer
RVR Labs Pvt Ltd
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 52-24-4 Triethylenethiophosphoramide 98%View Details
52-24-4 Triethylenethiophosphoramide 98%View Details
52-24-4 -
 52-24-4 98%View Details
52-24-4 98%View Details
52-24-4 -
 52-24-4 Triethylenethiophosphoramide 98%View Details
52-24-4 Triethylenethiophosphoramide 98%View Details
52-24-4 -
 ThioTEPA >95% CAS 52-24-4View Details
ThioTEPA >95% CAS 52-24-4View Details
52-24-4 -
 Thiotepa CAS 52-24-4View Details
Thiotepa CAS 52-24-4View Details
52-24-4 -
 Tri(aziridin-1-yl)phosphine sulfide (Thiotepa) 52-24-4 98%View Details
Tri(aziridin-1-yl)phosphine sulfide (Thiotepa) 52-24-4 98%View Details
52-24-4 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
