Triethylenediamine
Synonym(s):1,4-Diazabicyclo[2.2.2]octane;Triethylenediamine;TED;TMEM28;Transmembrane protein
- CAS NO.:280-57-9
- Empirical Formula: C6H12N2
- Molecular Weight: 112.17
- MDL number: MFCD00006689
- EINECS: 205-999-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
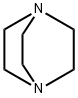
What is Triethylenediamine?
Description
DABCO, short for1,4-diazabicyclo[2.2.2]octane, is one of a handful of molecules better known by their acronyms than by their full names1. It is also frequently referred to as triethylenediamine, or TEDA.
DABCO has been known for at least 100 years, originally under the name quinolidine. In 1943, Otto Hromatka* and Eva Engel at the University of Vienna synthesized it via the cyclization of the dihydrobromide salt of 1-(2-bromoethyl)piperazine2 or the dihydrochloride salt of 1-(2-chloroethyl)piperazine3. It is commercially produced by the catalytic thermolysis of ethylenediamine or 2-hydroxyethylamine.
Because of its two unhindered tertiary amine groups, DABCO is a strong base (pKa1 of its conjugate acid = 3.0) and nucleophile. It is used as an alkaline catalyst in the production of polyurethane foams. As a Lewis base, it forms crystalline adducts with hydrogen peroxide and sulfur dioxide.
This year, DABCO was featured as the catalyst in two new syntheses. In April, Silong Xu and co-workers at Xi’an Jiaotong University (China) showed that it catalyzes [4 + 2] annulation reactions between 5-methylenehex-2-ynedioates and electron-deficient olefins such as 2-benzylidene-1H-indene-1,3-(2H)-dione to form spirocyclohexadiene adducts in yields up to 90% under mild conditions. Alternative catalysts gave poor yields or no reaction.
In July, Cunde Wang and colleagues at Yangzhou University (China) reported the use of DABCO to promote another set of cyclization reactions. In their process, substituted 2-amino-4H-chromen-4-ones and substituted 2,6-dibenzylidenecyclohexan-1-ones react to form 7,8,9,10-tetrahydro-12H-chromeno[2,3-b]quinolin-12-ones, also under mild conditions and in optimized yields of >80%. As with the Xu process, other basic catalysts gave significantly poorer yields.
1. Another is TEMPO, which will appear as a Molecule of the Week later this year.
2. CAS Reg. No. 89727-93-5.
3. CAS Reg. No. 34782-06-4.
Description
DABCO is a caged tertiary diamine, and is generally described as a highly nucleophilic amine. DABCO can serve as an organic base, complexing ligand, or a catalyst.
Chemical properties
Triethylenediamine also known as DABCO or TEDA, is a highly symmetrical molecule with a cage structure. The colorless extremely hygroscopic crystals is a highly nucleophilic tertiary amine base, which is used as a catalyst and reagent in polymerization and organic synthesis.
The Uses of Triethylenediamine
An anti-fade reagent shown to scavenge free-radicals due to flurochrome excitation.
The Uses of Triethylenediamine
1,4-Diazabicyclo[2.2.2]octane is used as polyurethane catalyst, Balis-Hillman reaction catalyst complexing ligand and lewis base. It finds use in dye lasers and in mounting samples for fluorescence microscopy and as anti-fade reagent shown to scavenge free radicals due to flurochrome excitation of fluorochromes. Further, it is an oxidation and polymerization catalyst.
What are the applications of Application
1,4-Diazabicyclo[2.2.2]octane is an anti-fade reagent shown to scavenge free-radicals due to flurochrome excitation
Definition
ChEBI: Triethylenediamine is an organic heterobicylic compound that is piperazine with an ethane-1,2-diyl group forming a bridge between N1 and N4. It is typically used as a catalyst in polymerization reactions. It has a role as a catalyst, a reagent and an antioxidant. It is a bridged compound, a tertiary amino compound, a saturated organic heterobicyclic parent and a diamine.
Preparation
Triethylenediamine can be produced from ethylenediamine or ethanolamine, diethanolamine, or diethylenetriamine with a variety of different catalysts.
Reactions
Triethylenediamine reacts virtually quantitatively with bromine to give a 1/1 adduct. With alkyl halides it forms quaternary salts, even in nonpolar solvents. Apart from its highly nucleophilic nature, triethylenediamine exhibits catalytic activity in base-catalyzed reactions.
General Description
Dabco?33-LV (Db) is a gelling catalyst and a bidentate ligand that forms a self-assembled monolayer (SAM) on a variety of substrates. It functionalizes the surface and immobilizes the surface atoms.
Hazard
Skin irritant.
Flammability and Explosibility
Flammable
Purification Methods
DABCO crystallises from 95% EtOH, pet ether or MeOH/diethyl ether (1:1). Dry it under vacuum over CaCl2 and BaO. It can be sublimed in vacuo, and readily at room temperature. It has also been purified by removal of water during azeotropic distillation of a *benzene solution. It is then recrystallised twice from anhydrous diethyl ether under argon, and stored under argon [Blackstock et al. J Org Chem 52 1451 1987]. [Beilstein 23/3 V 487.]
Properties of Triethylenediamine
| Melting point: | 156-159 °C(lit.) |
| Boiling point: | 174 °C |
| Density | 1.02 g/mL |
| vapor pressure | 2.9 mm Hg ( 50 °C) |
| refractive index | n |
| Flash point: | 198 °F |
| storage temp. | Store below +30°C. |
| solubility | 400g/l |
| form | Hygroscopic Crystals |
| appearance | White solid |
| appearance | hygroscopic white crystals |
| pka | 3.0, 8.7(at 25℃) |
| color | White to pale yellow |
| Water Solubility | 46 g/100 mL (26 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,9669 |
| BRN | 103618 |
| Stability: | Stable, but very hygroscopic. Incompatible with strong oxidizing agents, strong acids. Highly flammable. |
| CAS DataBase Reference | 280-57-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Triethylenediamine(280-57-9) |
| EPA Substance Registry System | 1,4-Diazabicyclo[2.2.2]octane (280-57-9) |
Safety information for Triethylenediamine
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H228:Flammable solids H302:Acute toxicity,oral H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P240:Ground/bond container and receiving equipment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Triethylenediamine
Triethylenediamine manufacturer
JSK Chemicals
CEFA CILINAS BIOTICS PVT LTD
ASM Organics
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran

You may like
-
 280-57-9 98%View Details
280-57-9 98%View Details
280-57-9 -
![1,4-Diazabicyclo[2.2.2]octane 98%](https://img.chemicalbook.in//ProductImageIndia/2024-03/Raw/60150b6f-9202-4e1e-97bc-f8dbe6bf29d2.png) 1,4-Diazabicyclo[2.2.2]octane 98%View Details
1,4-Diazabicyclo[2.2.2]octane 98%View Details -
![1,4-Diazabicyclo[2.2.2]octane CAS 280-57-9](https://img.chemicalbook.in//Content/image/CP5.jpg) 1,4-Diazabicyclo[2.2.2]octane CAS 280-57-9View Details
1,4-Diazabicyclo[2.2.2]octane CAS 280-57-9View Details
280-57-9 -
![1,4-Diazabicyclo[2.2.2]octane CAS 280-57-9](https://img.chemicalbook.in//Content/image/CP5.jpg) 1,4-Diazabicyclo[2.2.2]octane CAS 280-57-9View Details
1,4-Diazabicyclo[2.2.2]octane CAS 280-57-9View Details
280-57-9 -
 1,4 - DIAZABICYCLO (2.2.2) OCTANE For Synthesis CAS 280-57-9View Details
1,4 - DIAZABICYCLO (2.2.2) OCTANE For Synthesis CAS 280-57-9View Details
280-57-9 -
 Triethylenediamine CASView Details
Triethylenediamine CASView Details -
![1,4-Diazabicyclo[2.2.2]octane CAS 280-57-9](https://img.chemicalbook.in//Content/image/CP5.jpg) 1,4-Diazabicyclo[2.2.2]octane CAS 280-57-9View Details
1,4-Diazabicyclo[2.2.2]octane CAS 280-57-9View Details
280-57-9 -
 Dabco® 33-LV CAS 280-57-9View Details
Dabco® 33-LV CAS 280-57-9View Details
280-57-9
