CARBONYL SULFIDE
Synonym(s):Carbon oxysulfide
- CAS NO.:463-58-1
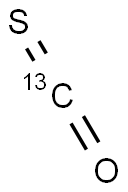
- Empirical Formula: COS
- Molecular Weight: 60.07
- MDL number: MFCD00011521
- EINECS: 207-340-0
- Update Date: 2025-12-10 11:56:18

What is CARBONYL SULFIDE?
Description
Carbonyl sulfide (COS) is a colorless gas with a sulfur-like odor. Like carbon dioxide (CO2), its molecular structure is linear; but unlike CO2?it is flammable. It decomposes slowly in water and more rapidly in the presence of base.
J. P. Cou?rbe described what he thought was COS in 1841, but the gas turned out to be a mixture of CO2?and hydrogen sulfide (H2S). In 1867, Hungarian Chemist Carl von Than characterized it correctly; he made it by the reaction between potassium thiocyanate (KSCN) and sulfuric acid (H2SO4), although other gases were coproduced.
For a simple molecule, COS has relatively few applications in research and manufacturing. But at ≈0.5 ppb, it is the most abundant sulfur-containing compound in Earth’s atmosphere, a circumstance that turns out to be surprisingly useful in Earth science.
About 10 years ago, J. Elliott Campbell, then at the University of California, Merced, and other scientists began to evaluate whether COS is a good surrogate for CO2?for tracking how much carbon is taken up by photosynthesis worldwide. Even though COS’s concentration in the atmosphere is smaller than that of CO2?by a factor of ≈106, its atmospheric “signal” is 6 times greater than CO2; and it avoids some of the complications that accompany CO2measurements.
Earlier this year, Campbell, now at the University of California, Santa Cruz, and about two dozen coauthors at multiple institutions reported that?COS is likely superior to CO2?for tracing photosynthesis activity worldwide?despite COS’s own complications. Their conclusion: “Pursuing multiple lines of evidence, including the COS technique, may yet provide a tractable path for addressing the pressing concern of carbon processes within the climate system.”
Description
Carbonyl oxysulfide is a colorless gas or coldliquid. Molecular weight=60.07. Boiling point=250℃.Flammable limits: LEL=12%; UEL=29%. HazardIdentification (based on NFPA-704 M Rating System):Health 3, Flammability 4, Reactivity 1.
Chemical properties
colourless gas with an unpleasant smell; cylinder
Chemical properties
Carbonyl sulfide is a colorless gas or cold liquid.
Occurrence
Carbonyl sulfide, COS, is now recognized as a component of the atmosphere at a tropospheric concentration of approximately 500 parts per trillion by volume, corresponding to a global burden of about 2.4 million tons. It is, therefore, a significant sulfur species in the atmosphere. It is possible that the HO• radicalinitiated oxidation of COS and carbon disulfide (CS2) would yield 8-12 million tons as S in atmospheric sulfur dioxide per year. Though this is a small yield compared to pollution sources, the HO•-initiated process could account for much of the SO2 burden in the remote troposphere.
Both COS and CS2 are oxidized in the atmosphere by reactions initiated by the hydroxyl radical. The initial reactions are
HO• + COS ® CO2 + HS• (11.10.1)
HO• + CS2 ® COS + HS• (11.10.2)
These reactions with hydroxyl radical initiate oxidation processes that occur through a series of atmospheric chemical reactions. The sulfur-containing products that are initially formed as shown by Reactions 11.10.1 and 11.10.2 undergo further reactions to sulfur dioxide and, eventually, to sulfate species.
The Uses of CARBONYL SULFIDE
carbonyl sulfide is use as a fumigant for durable commodities and structures was patented worldwide by Australia in 1992. It is effective on a wide range of pests, including the common stored product species at reasonable concentrations (less than 50 gm-3) and exposure times (1-5 days) . However, the egg stage of several insects showed tolerance to the fumigant. The other problems associated with the use of carbonyl sulfide include its high tainting odour on the treated products and reduction in the germination of seeds. Hydrogen sulphide, an impurity, present in fumigant product supply was reported to be responsible for the off-odour problem. Selective removal of hydrogen sulphide using absorbents like tertiary amine may solve the tainting issues with this fumigant.
The Uses of CARBONYL SULFIDE
Grain fumigant.
The Uses of CARBONYL SULFIDE
Carbonyl sulfide (COS) is a colorless, odorless (when pure)
relatively stable gas with a boiling point of -50°C.
There are limited commercial uses of COS. It is produced
only in small quantities and used for small-scale experimental
purposes and as an intermediate in the synthesis of organic
sulfur compounds, thiocarbamate herbicides, and alkyl
carbonates. Pesticide manufacturers are believed to be the largest
users of COS. Similar to CS2, research conducted by the Stored
Grain Research Laboratory at the Australia’s Commonwealth
Scientific and Industrial Research Organisation (CSIRO) has
shown COS to be an effective soil and grain fumigant for
controlling insects on crops such as wheat, barley, oats, and peas,
although it is not currently approved for this commercial use.
The use of COS as a fumigant for durable commodities and
structures was patented worldwide in 1992 by CSIRO Australia.
COS has the potential to replace methyl bromide, being phased
out due to its ozone depletion properties, in several of its
applications for durable commodities and also to be used as an
alternative to phosphine when there is a significant problem
with insect resistance.
Definition
ChEBI: A one-carbon compound in which the carbon atom is attached to an oxygen and a sulfur atom via double bonds.
General Description
CARBONYL SULFIDE is a colorless, poisonous, flammable gas with a distinct sulfide odor. The gas is toxic and narcotic in low concentrations and presents a moderate fire hazard. Under prolonged exposure to fire or intense heat the container may rupture violently or rocket. CARBONYL SULFIDE is used in the synthesis of organic thio compounds.
Air & Water Reactions
Highly flammable.
Reactivity Profile
CARBONYL SULFIDE is expected to react with vigor with strong oxidants.
Hazard
Narcotic in high concentrations. Flammable, explosive limits in air 12–28.5%. Central nervous system impairment.
Health Hazard
TOXIC; may be fatal if inhaled or absorbed through skin. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution.
Fire Hazard
Flammable; may be ignited by heat, sparks or flames. May form explosive mixtures with air. Vapors from liquefied gas are initially heavier than air and spread along ground. Vapors may travel to source of ignition and flash back. Some of these materials may react violently with water. Cylinders exposed to fire may vent and release toxic and flammable gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket. Runoff may create fire or explosion hazard.
Safety Profile
Poison by intraperitoneal route. Mildly toxic by inhalation. Narcotic in high concentration. An irritant. May liberate highly toxic hydrogen sulfide upon decomposition. A very dangerous fire hazard and moderate explosion hazard when exposed to heat or flame. Can react vigorously with oxidizing materials. To fight fire, stop flow of gas or use CO2, dry chemical, or water spray. When heated to decomposition it emits toxic fumes of CO. See also CARBONYLS and SULFIDES.
Potential Exposure
Carbon oxysulfide is an excellent source of usable atomic sulfur, therefore, it can be used in various chemical syntheses, such as the production of episulfides, alkenylthiols, and vinylicthiols. It is also used to make viscose rayon. It is probable that the largest source of carbon oxysulfide is as a by-product from various organic syntheses and petrochemical processes. Carbon oxysulfide is always formed when carbon, oxygen, and sulfur, or their compounds, such as carbon monoxide; carbon disulfide, and sulfur dioxide, are brought together at high temperatures. Hence, carbon, oxysulfide is formed as an impurity in various types of manufactured gases and as a by-product in the manufacture of carbon disulfide. Carbon oxysulfide is also often present in refinery gases.
First aid
Gas: Move victim to fresh air. Call emergencymedical care. Apply artificial respiration if victim is notbreathing. Do not use mouth-to-mouth method if victimingested or inhaled the substance; induce artificial respiration with the aid of a pocket mask equipped with a one-wayvalve or other proper respiratory medical device.Administer oxygen if breathing is difficult. Remove andisolate contaminated clothing and shoes. In case of contactwith substance, immediately flush skin or eyes with runningwater for at least 20 min. In case of contact with liquefiedgas, thaw frosted parts with lukewarm water. Keep victimwarm and quiet. Keep victim under observation. Effects ofcontact or inhalation may be delayed. Ensure that medicalpersonnel are aware of the material(s) involved and takeprecautions to protect themselves.Refrigerated liquid: Move victims to fresh air. Call emergency medical care. Apply artificial respiration if victim isnot breathing. Administer oxygen if breathing is difficult.Remove and isolate contaminated clothing and shoes. Incase of contact with substance, immediately flush skin oreyes with running water for at least 20 min. In case of contact with liquefied gas, thaw frosted parts with lukewarmwater. Keep victim warm and quiet. Keep victim underobservation. Effects of contact or inhalation may bedelayed. Ensure that medical personnel are aware of thematerial(s) involved and take precautions to protect themselves. If frostbite has occurred, seek medical attentionimmediately; do NOT rub the affected areas or flush themwith water. In order to prevent further tissue damage, doNOT attempt to remove frozen clothing from frostbittenareas. If frostbite has NOT occurred, immediately and thoroughly wash contaminated skin with soap and water.
Environmental Fate
Toxicity from exposure to COS is likely the result of its
decomposition to CO2 and H2S. H2S inhibits respiration at the
cellular level, causing methemoglobinemia, which inhibits the
cytochrome oxidase system, causing cytotoxic anoxia. In one
study, rats treated with acetazolamide, an inhibitor of carbonic
anhydrase, showed lower blood levels of H2S following exposure
to COS and exhibited decreased toxicity. H2S is believed to
be primarily responsible for many of the reported adverse
effects associated with exposure to COS.
COS reacts readily with ammonia and primary amines to
form ammonium thiocarbamate and amine salts of monothiocarbamic
acid, respectively. Reaction with two primary
amines may result in the formation of H2S and linking of the
two amines via a carbonyl group reaction, suggesting considerable
potential for protein cross-linking by COS in vivo, and
this has been proposed as a mechanism to explain occupational
neuropathy observed with CS2, and predicted for COS.
Storage
Color Code—Red Stripe: Flammability Hazard:Store separately from all other flammable materials. Priorto working with carbon oxysulfide you should be trained onits proper handling and storage. Before entering confinedspace where this chemical may be present, check to makesure that an explosive concentration does not exist. Carbonoxysulfide must be stored to avoid contact with bases andstrong oxidizers since violent reactions occur. Keep containers in a cool, well-ventilated area away from heat,flame, and sunlight. Metal containers involving the transferof=gallons or more of liquid carbon oxysulfide should begrounded and bonded. Drums must be equipped with selfclosing valves, pressure vacuum bungs, and flame arresters.Use only nonsparking tools and equipment, especially whenopening and closing containers of COS. Sources of ignition,such as smoking and open flames, are prohibited where carbon oxysulfide is used, handled, or stored. Procedures forthe handling, use and storage of cylinders should be in compliance with OSHA 1910.101 and 1910.169, as with therecommendations of the Compressed Gas Association.
Shipping
UN2204 Carbonyl sulfide, Hazard Class: 2.3; Labels: 2.3-Poisonous gas, 2.1-Flammable gas, Inhalation Hazard Zone C. It is a violation of transportation regulations to refill compressed gas cylinders without the express written permission of the owner. Cylinders must be transported in a secure upright position, in a well-ventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written permission of the owner.
Purification Methods
Purify the gas by scrubbing it through three consecutive fritted washing flasks containing conc NaOH at 0o (to remove HCN), and then through conc H2SO4 (to remove CS2) followed by a mixture of NaN3 and NaOH solution; or passed through traps containing saturated aqueous lead acetate, then through a column of anhydrous CaSO4. Then it is freeze-pumped repeatedly and distilled through a trap packed with glass wool and cooled to -130o (using an n-pentane slurry). It liquefies at 0o/12.5mm. Use stainless steel containers. The gas is stored over conc H2SO4. [Glemser in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 654 1963.] TOXIC
Toxicity evaluation
Most of the releases of COS to the environment are to air,
where it is believed to have a long residence time. Its half-life
in the atmosphere is estimated to be approximately 2 years.
It may be degraded in the atmosphere via a reaction with
photochemically produced hydroxyl radicals or oxygen,
direct photolysis, and other unknown processes related to the
sulfur cycle. Sulfur dioxide, a greenhouse gas, is ultimately
produced from these reactions. COS is relatively unreactive in
the troposphere, but direct photolysis may occur in the stratosphere. Also, plants and soil microorganisms have been
reported to remove COS directly from the atmosphere. Plants
are not expected to store COS.
COS is extremely mobile in soils. If released to soil, it will
volatilize quickly to the atmosphere (Koc= 88). It has a high
solubility in water and will not readily adsorb to soil particles,
sediment, or suspended organic matter. Therefore, COS is expected
to volatilize rapidly from soil and water or, depending on
volume, concentration, and site-specific characteristics (e.g., soil
type, depth to groundwater, temperature, and humidity), maybe
able to move rapidly through the ground and impact groundwater.
COS may be hydrolyzed in water to form H2S and CO2.
COS is also actively taken up by some plants and converted
to CS2; that is, the atmospheric pathways are reversed,
and soils may act as both a net source and a net sink for COS
depending on the concentration of COS and the characteristics
of the soil. COS is therefore accurately described as
a naturally occurring and widely distributed chemical found
or produced in the air, soils, live and decomposing vegetation,
and food.
Incompatibilities
Carbon oxysulfide can form explosive mixture with air. Incompatible with strong bases. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
Waste Disposal
Return refillable compressed gas cylinders to supplier. Dissolve in a combustible solvent, such as alcohol, benzene, etc. Burn in a furnace with afterburner and scrubber to remove SO2 .
Properties of CARBONYL SULFIDE
| Melting point: | −138 °C(lit.) |
| Boiling point: | −50 °C(lit.) |
| Density | 1.274; 2.4849 |
| vapor density | 2.1 (20 °C, vs air) |
| vapor pressure | 9034 mm Hg ( 21 °C) |
| refractive index | n 1.3785 |
| solubility | soluble in H2O, ethanol |
| form | gas |
| color | colorless |
| explosive limit | 11.9-29% |
| Odor Threshold | 0.055ppm |
| Water Solubility | mL/100mL H2O: 133.3 (0°C), 56.1 (20°C), 40.3 (30°C) [LAN05]; slowly decomposes in H2O [COT88]; soluble alcohol [HAW93] |
| Stability: | Stable. Corrosive to common metals when moisture is present. Reacts vigorously with oxidants. Flammable. Suck-back into cylinder may cause rupture. |
| EPA Substance Registry System | Carbonyl sulfide (463-58-1) |
Safety information for CARBONYL SULFIDE
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Gas Cylinder Compressed Gases GHS04  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H221:Flammable gases H280:Gases under pressure H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H330:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P403+P233:Store in a well-ventilated place. Keep container tightly closed. P410+P403:Protect from sunlight. Store in a well-ventilated place. |
Computed Descriptors for CARBONYL SULFIDE
| InChIKey | JJWKPURADFRFRB-UHFFFAOYSA-N |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


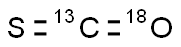
You may like
-
 463-58-1 Carbonyl Sulphide 98%View Details
463-58-1 Carbonyl Sulphide 98%View Details
463-58-1 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
