52-24-4
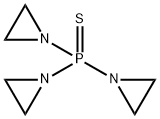
Product Name:
Triethylenethiophosphoramide
Formula:
C6H12N3PS
Synonyms:
Thio-TEPA;Tiofosfamid;Tiofosyl;Tiofozil;Tio-tef
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Tris(aziridinyl)phosphine sulfide is an odorless white crystalline solid. (NTP, 1992) |
|---|---|
| Color/Form | Crystals from pentane or ether |
| Odor | Faint odor |
| Boiling Point | Decomposes at 282 °F (NTP, 1992) |
| Melting Point | 124.7 °F (NTP, 1992) |
| Solubility | >28.4 [ug/mL] (The mean of the results at pH 7.4) |
| Vapor Pressure | 0.00845 [mmHg] |
| LogP | 0.53 |
| Stability/Shelf Life | Stable under recommended storage conditions. |
| Decomposition | When heated to decomposition if emits very toxic fumes of POx, SOx, and NOx. |
| Kovats Retention Index | 1522.3 |
| Other Experimental Properties | In dilute solution at pH 1 hydrogen sulfide is released (T/2, approx 3 hr) |
| Chemical Classes | Pesticides -> Insect Sterilants |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H300:Acute toxicity,oral H350:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. |
COMPUTED DESCRIPTORS
| Molecular Weight | 189.22 g/mol |
|---|---|
| XLogP3 | 0.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 189.04895557 g/mol |
| Monoisotopic Mass | 189.04895557 g/mol |
| Topological Polar Surface Area | 41.1 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 194 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Tris(aziridinyl)phosphine sulfide is an odorless white crystalline solid. (NTP, 1992)
