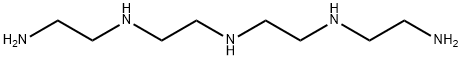
Triethylenetetramine
Synonym(s):DEH 24;EPH 925;N,N′-Bis(2-aminoethyl)-1,2-ethanediamine;TETA;Triethylenetetramine
- CAS NO.:112-24-3
- Empirical Formula: C6H18N4
- Molecular Weight: 146.23
- MDL number: MFCD00008169
- EINECS: 203-950-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02

What is Triethylenetetramine?
Absorption
TETA is poorly absorbed from the gastrointestinal tract with an oral bioavailability ranging from 6% to 18%. TETA has the potential to chelate non-copper cations in mineral supplements and other oral drugs, resulting in altered drug absorption; thus, TETA should be administered at least one hour apart from these medications.
The median Tmax ranges from 1.25 to 2 hours. Mean Cmax (± SD) of triethylenetetramine (TETA) was 2030 ± 981 ng/mL following oral administration of 900 mg TETA and 3430 ± 1480 ng/mL following administration of 1500 mg TETA. The systemic exposure (AUC) of TETA increased in a dose-proportional manner over the range of 900 mg to 1500 mg TETA. The mean AUCinf (± SD) was 9750 ± 4910 ngxh/mL at 900 mg and 17200 ± 9470 ngxh/mL at 1500 mg.
Toxicity
The oral LD50 was 2500 mg/kg in rats. The dermal LD50 was 550 mg/kg in rabbits.
Occasional cases of trientine overdose have been reported. A large overdose of 60 g of trientine hydrochloride resulted in nausea, vomiting, dizziness, mild acute kidney injury, mild hypophosphatemia, low serum zinc, and low serum copper: the patient recovered following intravenous hydration and supportive measures. There is no antidote for an acute overdose from trientine. Chronic use of trientine at dosages above the maximum recommended dosage has resulted in sideroblastic anemia.
Description
Cross sensitivity is possible with diethylenetriamine and diethylenediamine.
Description
The linear polyamine triethylenetetramine is a high–boiling point, oily liquid that was first prepared in 1890 by A. W. von Hoffmann. He built on the synthesis of ethylenediamine by using condensation reactions between chloramines. Today, triethylenetetramine is made by heating ethylenediamine or ethanolamine with ammonia and then separating it from the product mixture.
Triethylenetetramine’s primary commercial use is curing epoxy resins. It is also used in analytical procedures for determining nickel and copper; it is a lubricating oil additive; and in medicine, along with penicillamine, it is used to remove copper from the body as a treatment for Wilson’s disease.
In 2009, P. Anzenbacher, Jr., and M. A. Palacios at Bowling Green State University (OH) used triethylenetetramine as a reagent in “attoreactors” made from electrospun polyurethane nanofibers. (An attoliter [aL] is 10–15liters.) Combining fibers doped with the tetramine with others doped with dansyl chloride and heating the fiber structure produced fluorescent dansylamides on the zeptomole scale (10–21?mol). A 5-aL reactor contains only ≈1500 molecules!
Chemical properties
Triethylenetetramine is a corrosive liquid.
The Uses of Triethylenetetramine
Triethylenetetramine is used in synthesis of detergents, softeners, and dyestuffs; manufacture of pharmaceuticals; vulcanization accelerator of rubber; thermo setting resin; epoxy curing agent; lubricating-oil additive; analytical reagent for Cu, Ni; chelating agent; treatment of Wilson's disease.
The Uses of Triethylenetetramine
Triethylenetetramine is a selective CuII-chelator; crosslinking agent. Triethylenetetramine is undergoing trials for the treatment of heart failure in patients with diabetes.
The Uses of Triethylenetetramine
Triethylenetetramine is used as an amine hardener in epoxy resin of the bisphenol A type.
Background
Triethylenetatramine (TETA), also known as trientine, is a potent and selective copper (II)-selective chelator. It is a structural analog of linear polyamine compounds, spermidine and spermine. TETA was first developed in Germany in 1861 and its chelating properties were first recognized in 1925. Initially approved by the FDA in 1985 as a second-line treatment for Wilson's disease, TETA is currently indicated to treat adults with stable Wilson’s disease who are de-coppered and tolerant to penicillamine.
TETA has been investigated in clinical trials for the treatment of heart failure in patients with diabetes.
Indications
Triethylenetetramine is a copper chelator indicated for the treatment of adult patients with stable Wilson’s disease who are de-coppered and tolerant to penicillamine.
What are the applications of Application
Triethylenetetramine is a selective Cu(II) chelator and crosslinker
Production Methods
TETA is manufactured by reacting ethylene dichloride and ammonia under controlled conditions.
Definition
ChEBI: 2,2,2-tetramine is a polyazaalkane that is decane in which the carbon atoms at positions 1, 4, 7 and 10 are replaced by nitrogens. It has a role as a copper chelator. It is a tetramine and a polyazaalkane.
brand name
Syprine (Merck).
General Description
A yellowish liquid. Less dense than water. Combustible, though may be difficult to ignite. Corrosive to metals and tissue. Vapors heavier than air. Toxic oxides of nitrogen produced during combustion. Used in detergents and in the synthesis of dyes, pharmaceuticals and other chemicals.
Chemical properties
Triethylenetetramine (TETA) is hygroscopic, corrosive, and has a strong ammoniacal odor. With water a crystalline hydrate is formed. Like DETA, it is completely miscible with water and many polar organic solvents, but less so with lipids; with CCl4 a violent reaction occurs. Its four pKa values are 3.32, 6.67, 9.20, and 9.92. Technical-grade TETA is sometimes available as a distillation cut that also contains branched isomers and cyclic compounds.
Reactivity Profile
Triethylenetetramine is a strong base; reacts violently with strong oxidants; attacks aluminum, zinc, copper and its alloys. Handling Chemicals Safely 198. p. 934).
Health Hazard
Vapors from hot liquid can irritate eyes and upper respiratory system. Liquid burns eyes and skin. May cause sensitization of skin.
Fire Hazard
Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.
Flammability and Explosibility
Non flammable
Contact allergens
Triethylenetetramine is used as an amine hardener in epoxy resins of the bisphenol A type. Cross-sensitivity is possible with diethylenetriamine and diethylenediamine.
Pharmacokinetics
Triethylenetetramine (TETA) is a selective copper(II) chelator that works to promote urinary copper excretion. It was shown to reduce excess body copper storage and ameliorate symptoms of Wilson’s disease. In rats with diabetes mellitus, intravenous administration of TETA led to a dose-dependent increase in urinary copper excretion.
In preliminary studies, TETA was shown to ameliorate left ventricular hypertrophy in both human and animal subjects with diabetes. In animal models, TETA was also shown to reverse manifestations of diabetic nephropathy, including nephromegaly, renal fibrosis, glomerulosclerosis, and albuminuria, without lowering hyperglycemia. This finding may be explained by TETA chelating copper cations, which are pro-oxidant and activate pathways that produce excessive reactive oxygen species (ROS) that cause tissue injury. TETA was shown to possess anti-angiogenesis properties, as copper is an essential element for angiogenesis in cancer cells. TETA was shown to inhibit telomerase, suggesting that it may exhibit an inhibitory effect or cytotoxicity on tumor growth. Based on these early findings, TETA has been studied for its anticancer effects.
Safety Profile
Poison by intravenous route. Moderately toxic by ingestion and skin contact. An experimental teratogen. Experimental reproductive effects. Mutation data reported. A corrosive irritant to skin, eyes, and mucous membranes. Causes skin sensitization. Combustible when exposed to heat or flame. Ignites on contact with cellulose nitrate of high surface area. Can react with oxidizing materials. To fight fire, use CO2, dry chemical, alcohol foam. When heated to decomposition it emits toxic fumes of NOx.
Toxicology
Triethylenetetramine may induce lung edema on inhalation of its vapors. The compound is also an effective skin sensitizer. Following repeated dosing (ca. 50 mg each, 17 – 55 times) onto the skin of pregnant and nonpregnant guinea pigs, significant amounts were absorbed by the strongly irritated skin, leading to toxic effects in the kidneys, liver, brain, and placenta and causing abortion. Triethylenetetramine proved to be a mutagen in vitro but not in vivo.
Carcinogenicity
TETA was mutagenic in bacterial assays and was positive in sister chromatid exchanges and unscheduled DNA synthesis tests in vitro.8 It was not clastogenic in the mouse micronucleus test in vivo after oral or intraperitoneal administration.
Metabolism
The majority of absorbed TETA is extensively metabolized into acetyl-metabolites. TETA undergoes acetylation mediated by diamine acetyltransferase, also known as spermidine/spermine N1-acetyltranferase, to form two major active metabolites, N1-acetyltriethylenetetramine (MAT) and N1,N10-diacetyltriethylenetetramine (DAT). The chelating activity of MAT is significantly lower than that of TETA.
Purification Methods
Dry the amine with sodium, then distil it under a vacuum. Further purification has been via the nitrate or the chloride salts. For example, Jonassen and Strickland [J Am Chem Soc 80 312 1958] separated TRIEN from admixture with TREN (38%) by solution in EtOH, cooling to approximately 5o in an ice-bath and adding conc HCl dropwise from a burette, keeping the temperature below 10o, until all of the white crystalline precipitate of TREN.HCl (see p 191) had formed and was removed. Further addition of HCl then precipitated thick, creamy white TRIEN.HCl (see below) which was crystallised several times from hot water by adding an excess of cold EtOH. The crystals were finally washed with Me2CO, then Et2O and dried in a vacuum desiccator. [Beilstein 4 H 255, 4 II 695, 4 III 542, 4 IV 1242.]
Properties of Triethylenetetramine
| Melting point: | 12 °C(lit.) |
| Boiling point: | 266-267 °C(lit.) |
| Density | 0.982 g/mL at 25 °C(lit.) |
| vapor density | ~5 (vs air) |
| vapor pressure | <0.01 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 290 °F |
| storage temp. | Store below +30°C. |
| solubility | alcohol: soluble |
| form | Slightly viscous yellow
liquid; commercially available form is 95–98%
pure, and impurities include linear, branched,
and cyclic isomers. |
| pka | pK1:3.32(+4);pK2:6.67(+3);pK3:9.20(+2);pK4:9.92(+1) (20°C) |
| color | Yellowish liquid or oil |
| PH | 10-11 (10g/l, H2O, 20℃) |
| explosive limit | 0.7-7.2%(V) |
| Water Solubility | SOLUBLE |
| FreezingPoint | 12℃ |
| Sensitive | Moisture Sensitive |
| Merck | 14,9663 |
| BRN | 605448 |
| Exposure limits | ACGIH: TWA 1 ppm (Skin) NIOSH: TWA 1 ppm(4 mg/m3) |
| Stability: | Incompatible with strong oxidizing agents, strong acids. |
| CAS DataBase Reference | 112-24-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Triethylenetetramine(112-24-3) |
| EPA Substance Registry System | Triethylenetetramine (112-24-3) |
Safety information for Triethylenetetramine
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H312:Acute toxicity,dermal H314:Skin corrosion/irritation H317:Sensitisation, Skin H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P272:Contaminated work clothing should not be allowed out of the workplace. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Triethylenetetramine
Triethylenetetramine manufacturer
JSK Chemicals
Triveni Interchem Private Limited (Group Of Triveni Chemicals)
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 TRIETHYLENE TETRAMINE (TETA) 99%View Details
TRIETHYLENE TETRAMINE (TETA) 99%View Details -
 Triethylene Tetramine 99%View Details
Triethylene Tetramine 99%View Details -
 Triethylenetetramine (mix. of isomers) pure CAS 112-24-3View Details
Triethylenetetramine (mix. of isomers) pure CAS 112-24-3View Details
112-24-3 -
 Triethylene Tetramine TetaView Details
Triethylene Tetramine TetaView Details
112-24-3 -
 TriethylenetetramineView Details
TriethylenetetramineView Details
112-24-3 -
 Industrial Triethylenetetramine TetaView Details
Industrial Triethylenetetramine TetaView Details
112-24-3 -
 200 L Triethylenetetramine ChemicalView Details
200 L Triethylenetetramine ChemicalView Details
112-24-3 -
 Tri Ethylene TetramineView Details
Tri Ethylene TetramineView Details
112-24-3
