1,4,7,10,13-Pentaazatridecane
Synonym(s):1,4,7,10,13-Pentaazatridecane;TEPA;Tetraethylene pentamine;Tetrene
- CAS NO.:112-57-2
- Empirical Formula: C8H23N5
- Molecular Weight: 189.3
- MDL number: MFCD00008168
- EINECS: 203-986-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
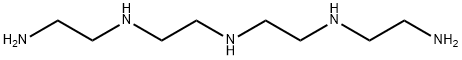
What is 1,4,7,10,13-Pentaazatridecane?
Chemical properties
colourless to green-yellow viscous liquid
Chemical properties
Tetraethylenepentamine is a yellow, viscous liquid.
The Uses of 1,4,7,10,13-Pentaazatridecane
Solvent for sulfur, acid gases, and various resins and dyes; saponifying agent for acidic materials; manufacture of synthetic rubber; dispersant in motor oils; intermediate for oil additives.
Definition
ChEBI: Tetraethylenepentamine is a polyazaalkane. It has a role as a copper chelator.
General Description
A viscous liquid. Slightly less dense than water. Vapors heavier than air. Corrosive to the eyes, skin, mouth, throat and stomach. Vapors irritate the eyes and corrosive to the upper respiratory tract. Vapors may irritate the eyes. Flash point 325°F.
Air & Water Reactions
Soluble in water. Hygroscopic.
Reactivity Profile
1,4,7,10,13-Pentaazatridecane is hygroscopic. 1,4,7,10,13-Pentaazatridecane can react with oxidizing materials and strong acids. 1,4,7,10,13-Pentaazatridecane may attack some forms of plastics.
Hazard
Strong irritant to eyes and skin.
Health Hazard
Inhalation may cause nausea and slight irritation; compound is a sensitizer, and prolonged contact may cause asthma. Ingestion can cause burns of mouth, esophagus, and possibly stomach. Contact with eyes or skin may cause burns. Repeated skin contact may cause dermatitis.
Fire Hazard
Special Hazards of Combustion Products: Ammonia and toxic oxides of nitrogen may form in fires.
Safety Profile
Poison by ingestion and intravenous routes. Moderately toxic by skin contact. Mutation data reported. A corrosive irritant to skin, eyes, and mucous membranes. Combustible when exposed to heat or flame. Can react with oxidizing materials. To fight fire, use CO2, dry chemical. When heated to decomposition it emits toxic fumes of NOx.
Potential Exposure
Tetraethylenepentamine is used as a solvent for resins and dyes, in manufacture of synthetic rubber; and intermediate for oil additives; in papermaking.
Shipping
UN2320 Tetraethylenepentamine, Hazard class: 8; Labels: 8-Corrosive material.
Purification Methods
Distil the amine under vacuum. Also purify via its penta hydrochloride, nitrate or sulfate. Jonassen, Frey and Schaafsma [J Phys Chem 61 504 1957] cooled a solution of 150g of the base in 300mL of 95% EtOH, and added dropwise 180mL of conc HCl, keeping the temperature below 20o. The white precipitate was filtered off, crystallised three times from EtOH/water, then washed with diethyl ether and dried by suction. Reilley and Holloway [J Am Chem Soc 80 2917 1958], starting with a similar solution cooled to 0o, added slowly (keeping the temperature below 10o) a solution of 4.5g-moles of HNO3 in 600mL of aqueous 50% EtOH (also cooled to 0o). The precipitate was filtered by suction, recrystallised five times from aqueous 5% HNO3, then washed with acetone and absolute EtOH and dried at 50o. [For purification via the sulfate see Reilley and Vavoulis (Anal Chem 31 243 1959), and for an additional purification step using the Schiff base with benzaldehyde see Jonassen et al. J Am Chem Soc 79 4279 1957]. [Beilstein 4 IV 1244.]
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. This chemical is strongly alkaline; reacts with acids.
Properties of 1,4,7,10,13-Pentaazatridecane
| Melting point: | -40 °C (lit.) |
| Boiling point: | 340 °C |
| Density | 0.998 g/mL at 25 °C (lit.) |
| vapor density | 6.53 (vs air) |
| vapor pressure | <0.01 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 365 °F |
| storage temp. | Store below +30°C. |
| solubility | 6540g/l |
| form | Liquid |
| pka | pK1:2.98(+5);pK2:4.72(+4);pK3:8.08(+3);pK4:9.10(+2);pK5:9.67(+1) (25°C,) |
| color | Clear |
| PH | 11.8 (20g/l, H2O, 20℃) |
| Odor | ammonia odor |
| explosive limit | 0.1-15%(V) |
| Water Solubility | SOLUBLE |
| Sensitive | air sensitive |
| BRN | 506966 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents, mineral acids, halogenated hydrocarbons, hydrogen peroxide. |
| CAS DataBase Reference | 112-57-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Tetraethylenepentamine(112-57-2) |
| EPA Substance Registry System | Tetraethylenepentamine (112-57-2) |
Safety information for 1,4,7,10,13-Pentaazatridecane
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H317:Sensitisation, Skin H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 1,4,7,10,13-Pentaazatridecane
1,4,7,10,13-Pentaazatridecane manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 TETRAETHYLENE PENTAMINE (TEPA) 99%View Details
TETRAETHYLENE PENTAMINE (TEPA) 99%View Details -
 112-57-2 98%View Details
112-57-2 98%View Details
112-57-2 -
 Tetraethylene Pentamine CASView Details
Tetraethylene Pentamine CASView Details -
 Tetraethylene Pentamine CASView Details
Tetraethylene Pentamine CASView Details -
 Tetraethylenepentamine (mixture of branched chain isomers and cyclic compounds) CAS 112-57-2View Details
Tetraethylenepentamine (mixture of branched chain isomers and cyclic compounds) CAS 112-57-2View Details
112-57-2 -
 Tetraethylene pentamine CAS 112-57-2View Details
Tetraethylene pentamine CAS 112-57-2View Details
112-57-2 -
 Tetraethylene PentamineView Details
Tetraethylene PentamineView Details
112-57-2 -
 200 Kg Tetraethylene Pentamine TepaView Details
200 Kg Tetraethylene Pentamine TepaView Details
112-57-2
