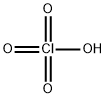
Ammonium perchlorate
- CAS NO.:7790-98-9
- Empirical Formula: ClH4NO4
- Molecular Weight: 117.49
- MDL number: MFCD00011421
- EINECS: 232-235-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
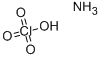
What is Ammonium perchlorate?
Description
Ammonium perchlorate is also shock-sensitive and may explode when exposed to heat or by spontaneous chemical reaction. This is the material that was involved in the explosion at the Pepcon plant in Henderson, Nevada. It is also used in the production of explosives, pyrotechnics, etching and engraving, and jet and rocket propellants.
Chemical properties
Ammonium perchlorate NH4CI04, is a white solid, soluble, formed by reaction of NH40H and perchlorate acid, and then evaporating. Used in explosives and pyrotechnics.
The Uses of Ammonium perchlorate
Perchlorate is a soluble oxychloro anion most commonly used as a solid salt in the form of ammonium perchlorate, potassium perchlorate, lithium perchlorate, or sodium perchlorate, all of which are highly soluble. Ammonium perchlorate is the most widely used perchlorate compound. In their pure forms, these salts are white or colorless crystals or powders. Perchlorate salts dissolve in water and readily move from surface to groundwater. Perchlorate is known to originate from both natural and man-made sources.
The most common uses for ammonium perchlorate are in explosives, military munitions, and rocket propellants. In addition, perchlorate salts are used in a wide range of nonmilitary applications, including pyrotechnics and fireworks, blasting agents, solid rocket fuel, matches, lubricating oils, nuclear reactors, air bags, and certain types of fertilizers.
The Uses of Ammonium perchlorate
Ammonium perchlorate is used to make rocket propellants, explosives, pyrotechnics, as an etching and engraving agent, and in analytical chemistry.
General Description
A white, crystalline solid or powder. Classified as a division 1.1 explosive if powdered into particles smaller than 15 microns in diameter or if powdered into larger particles but thoroughly dried. Does not readily burn, but will burn if contaminated by combustible material. May explode under prolonged exposure to heat or fire. Used to make rocket propellants, explosives, pyrotechnics, as an etching and engraving agent, and in analytical chemistry.
Air & Water Reactions
Water soluble.
Reactivity Profile
AMMONIUM PERCHLORATE is a strong oxidizing agent. Decomposes at 130°C and explodes at 380°C [Mellor 2 Supp. 1:608 1956]. Explosions have occurred in propellant formulations containing AMMONIUM PERCHLORATE to which ferrocene has been added as a burning rate catalyst. Although the cause was not been definitely established, AMMONIUM PERCHLORATE was most probably frictional heating from dragging a spatula through the mixture [ASESB Expl. Report 211 1966]. Can explode when mixed with sugar, charcoal or on contact with hot copper pipes. Becomes impact-sensitive when contaminated by powdered carbon, ferrocene, sulfur, or other reducing materials such as organic matter or powdered metals.
Hazard
Strong oxidizing agent; ignites violently with combustibles. Shock sensitive; may explode when exposed to heat or by spontaneous chemical reaction. Sensitive, high explosive when contaminated with reducing materials. Skin irritant.
Health Hazard
Irritating to skin and mucous membranes.
Flammability and Explosibility
Non flammable
Safety Profile
moderately toxic by ingestion and parented routes. Flammable when exposed to heat or flame or by spontaneous chemical reaction with reducing materials. A very powerful oxidizer that has caused explosions in industry. Ignites violently with combustibles. Severe explosion hazard; decomposes at 130' and explodes at 380'. When contaminated by powdered carbon, ferrocene, S, organic matter, powdered metals, nitryl perchlorate, potassium periodate, potassium permanganate, it becomes impact sensitive. Potentially explosive reactions with carbon (above 240℃), dichromium trioxide (at 270℃), cadmium oxide (at 260℃), zinc oxide (at 200°C), copper chromite, copper oxide, iron oxide, potassium permanganate, potassium dichromate, mono-, di-, tri-, or tetra-methylammonium perchlorates, metal perchlorates (e.g., lithium perchlorate, zinc perchlorate), nitrophenol-formaldehyde polymer. Mixtures with aluminum or copper burn violently when ignited. Mixtures with ethylene dinitrate ignite when stored at 60℃. When heated to decomposition it emits toxic fumes of NH3 and Cl-. See also PERCHLORATES and EXPLOSIVES, HIGH.
Purification Methods
It is recrystallised twice from distilled water (2.5mL/g) between 80o and 0o, and dried in a vacuum desiccator over P2O5. Drying at 110o might lead to slow decomposition to the chloride. POTENTIALLY EXPLOSIVE.
Properties of Ammonium perchlorate
| Melting point: | °Cd ec.) |
| Density | 1.95 g/mL at 25 °C(lit.) |
| vapor pressure | 0Pa at 25℃ |
| refractive index | 1.482 |
| solubility | soluble in methanol; slightly soluble in ethanol,
acetone; insoluble in ethyl ether |
| form | Crystalline |
| color | White |
| Specific Gravity | 1.95 |
| Water Solubility | Freely soluble in water. Soluble in methanol. Slightly soluble in ethanol, acetone. Insoluble in ether, ethyl acetate |
| Merck | 14,540 |
| Stability: | Explosive when mixed with combustible material. Incompatible with organics, paper, wood shavings, etc. May explode if heated under confinement or as a result of friction. Incompatible with metals, reducing agents, strong acids. |
| CAS DataBase Reference | 7790-98-9(CAS DataBase Reference) |
| EPA Substance Registry System | Ammonium perchlorate (7790-98-9) |
Safety information for Ammonium perchlorate
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H271:Oxidising liquids;Oxidising solids H319:Serious eye damage/eye irritation H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P220:Keep/Store away from clothing/…/combustible materials. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P370+P378:In case of fire: Use … for extinction. P371+P380+P375:in case of major fire and large quantities: Evacuate area. Fight fire remotely due to the risk of explosion. |
Computed Descriptors for Ammonium perchlorate
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
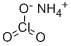
AMMONIUM PERCHLORATE](https://img.chemicalbook.in/StructureFile/ChemBookStructure1/GIF/CB0705891.gif)


You may like
-
 Ammonium perchlorate, GR 98%+ CAS 7790-98-9View Details
Ammonium perchlorate, GR 98%+ CAS 7790-98-9View Details
7790-98-9 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1