Tolmetin sodium
Synonym(s):1-Methyl-5-(p-toluoyl)pyrrole-2-acetic acid sodium salt dihydrate;Tolmetin sodium salt dihydrate
- CAS NO.:35711-34-3
- Empirical Formula: C15H14NNaO3
- Molecular Weight: 279.27
- MDL number: MFCD00079607
- EINECS: 252-687-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
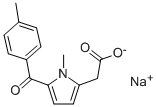
What is Tolmetin sodium?
Originator
Tolectin,McNeil,US,1976
The Uses of Tolmetin sodium
Tolmetin Sodium is a non-steroidal anti-inflammatory drug.
The Uses of Tolmetin sodium
Tolectin (Ortho-McNeil).
Definition
ChEBI: Tolmetin sodium is an organic sodium salt that is the monosodium salt of tolmetin. Used in the form of its dihydrate as a nonselective nonsteroidal anti-inflammatory drug. It has a role as an EC 1.14.99.1 (prostaglandin-endoperoxide synthase) inhibitor and a non-steroidal anti-inflammatory drug. It contains a tolmetin(1-).
Manufacturing Process
5-(p-Toluoyl)-1-methylpyrrole-2-acetonitrile - To a cooled suspension of 26.6 g
(0.2 mol) aluminum chloride in 80 ml dichloroethane is added dropwise 30.8 g
(0.2 mol) p-toluoyl chloride. The resulting solution is added dropwise to a
solution of 1-methylpyrrole-2-acetonitrile in 80 ml dichloroethane cooled
externally with an ice bath. After the addition, the resulting solution is stirred
at room temperature for 20 minutes and then refluxed for 3 minutes. The
solution is poured into ice acidified with dilute hydrochloric acid. The organic
and aqueous fractions are separated. The aqueous fraction is extracted once
with chloroform.
The organic fractions are combined and washed successively with N,N_x0002_dimethyl-1,3-propanediamine, dilute hydrochloric acid, saturated sodium
bicarbonate solution and saturated sodium chloride solution. The organic
fraction is dried over anhydrous magnesium sulfate. The solvent is then
evaporated off. Upon trituration of the residue with methanol, a solid
crystallizes, 5-(p-toluoyl)-1-methylpyrrole-2-acetonitrile, which is removed by
filtration and purified by recrystallization from benzene.
Additional product is isolated from the mother liquors which are combined,
concentrated in vacuo and the resulting oily residue column chromatographed
on neutral alumina using hexane, benzene and ether as successive solvents.
The product is isolated by concentrating in vacuo the first few major
compound-bearing fractions (10% ether in benzene). The solids are combined
and recrystallized from methanol and then from benzene-hexane, melting
point 102°C to 105°C.
5-(p-Toluoyl)-1-methylpyrrole-2-acetic acid - A solution of 3.67 g (0.015 mol)
of 5-(p-toluoyl)-1-methylpyrrole-2-acetonitrile, 24 ml of 1 N sodium hydroxide
and 50 ml of 95% ethanol is stirred and refluxed for 24 hours.
The resulting solution is poured into ice acidified with dilute hydrochloric acid.
A white solid precipitates which is extracted into ether. The ether phase is
washed with a saturated solution of sodium chloride and dried over anhydrous
magnesium sulfate. The solvent is evaporated and a white solid, 5-(p-toluoyl)-
1-methylpyrrole-2-acetic acid is obtained which is recrystallized twice from
isopropanol, melting point 155°C to 157°C.
Therapeutic Function
Antiinflammatory
Pharmacokinetics
Tolmetin sodium is rapidly and almost completely absorbed on oral administration, with peak plasma levels being attained within the first hour of administration. It has a relatively short plasma half-life of approximately 1 hour because of extensive first-pass metabolism, involving hydroxylation of the p-methyl group to the primary alcohol, which is subsequently oxidized to the dicarboxylic acid.
Clinical Use
Tolmetin is synthesized straightforwardly from 1-methylpyrrole. It was introduced in the United States in 1976 and like other NSAIDs, inhibits prostaglandin biosynthesis. Tolmetin, however, also inhibits polymorph migration and decreases capillary permeability. Its anti-inflammatory activity, as measured in the carrageenan-induced rat paw edema and cotton pellet granuloma assays, is intermediate between those of phenylbutazone and indomethacin.
Side Effects
The most frequently adverse reactions are those involving the GI tract (e.g., abdominal pain, discomfort, and nausea) but appear to be less than those observed with aspirin. The CNS effects (e.g., dizziness and drowsiness) also are observed. Few cases of overdosage have been reported, but in such cases, recommended treatment includes elimination of the drug from the GI tract by emesis or gastric lavage and elimination of the acidic drug from the circulatory system by enhancing alkalinization of the urine with sodium bicarbonate.
Metabolism
This metabolite is inactive in standard in vivo anti-inflammatory assays. The free acid (pKa = 3.5) is highly bound to plasma proteins (99%), and excretion of tolmetin and its metabolites occurs primarily in the urine.Approximately 15 to 20% of an administered dose is excreted unchanged and 10% as the glucuronide conjugate of the parent drug. Conjugates of the dicarboxylic acid metabolite account for the majority of the remaining administered drug.
Safety information for Tolmetin sodium
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8