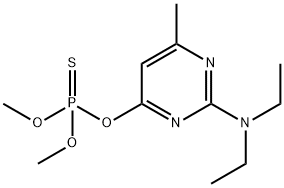
Tolclofos-methyl
Synonym(s):O-(2,6-Dichloro-4-methylphenyl) O,O-dimethyl phosphorothioate
- CAS NO.:57018-04-9
- Empirical Formula: C9H11Cl2O3PS
- Molecular Weight: 301.13
- MDL number: MFCD00078741
- EINECS: 260-515-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
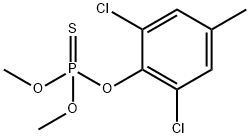
What is Tolclofos-methyl?
The Uses of Tolclofos-methyl
Tolclofos-methyl is a non-systemic organophosphorus fungicide with both protective and curative activities that control soil-borne diseases caused by Rhizoctonia solani, Corticium rolfsii, Tphula incamata and Typhula ishikariensis in/on potatoes, sugar beet, cotton and peanuts.
The Uses of Tolclofos-methyl
Agricultural fungicide.
Definition
ChEBI: An organic thiophosphate that is 2,6-dichloro-4-methylphenol in which the hydrogen of the hydroxy group group has been replaced by a dimethoxyphosphorothioyl group. Tolclofos-methyl is a phospholipid biosynthesis inhibitor and fungicide that is used for co trolling soil-borne diseases caused by Typhula incarnata, Corticium rolfsii, Typhula ishikariensis, and Rhizoctonia solani.
Metabolic pathway
Tolclofos-methyl underwent common degradation and metabolic pathways in water, soil, plants and animals. These reactions are well documented for most organophosphorus compounds and include oxidative desulfuration, hydroxylation/oxidation of the 4-methyl group to the alcohol and carboxylic acid, cleavage of the P-O-aryl linkage, O-demethylation and conjugation. In addition to the above reactions, photolytic isomerisation to the thionate (P=S) was also observed. A schematic presentation of the primary metabolic pathways for tolclofosmethyl is illustrated in Scheme 1.
Degradation
[14C-phenyl]Tolclofos-methyl(1) was stable to hydrolytic degradation at
pH 5, 7 and 9 at 22°C with DT50 values of 139, 417 and 238 days,
respectively. Higher temperature led to a more rapid hydrolysis and two
hydrolysis products were detected. O-Demethylation and oxidative desulfuration
were the major reactions to yield 2,6-dichloro-p-tolyl methyl
hydrogen phosphorothioate (2) and toclofos-methyl oxon (O-2,6-dichlorop-
tolyl O,O-dimethyl phosphate, 3), respectively. Cleavage of the P-O-aryl
linkage yielded 2,6-dichloro-4-methylphenol(4) as a minor degradation
product (WHO, 1994).
Tolclofos-methyl degraded in water under natural sunlight irradiation
with DT50 values of 44 days (in distilled water), 15-28 days (in natural
river and pond water) and less than 2 days in 2% acetone/water. The
major degradation reactions included oxidative desulfuration to yield
compound 3 and O-demethylation to yield compound 2. The major
photodegradation products in river and pond waters and soil thinlayer
surfaces were compounds 2, 3, 4 and the O-demethylated 3 (2,6-
dichloro-p-tolyl methyl hydrogen phosphate, 5) (Mikami et al., 1984).
In acetone solution, demethylation of the isomerisation product [2,6-
dichloro-p-tolyl O,S-dimethyl phosphorothioate (6)] yielded 2,6-dichlorop-
tolyl S-methyl hydrogen phosphorothioate (7) and 2,6-dichloro-p-tolyl
dihydrogen phosphate (8) as the major photodegradates (Mikami et al.,
1984).
Toxicity evaluation
Its acute oral toxicity is very low in comparison with a thio-ester type of other organophosphorus fungicides (edifenphos, iprobenfos, and pyrazophos).
Properties of Tolclofos-methyl
| Melting point: | 78-80°C |
| Boiling point: | 338.5±52.0 °C(Predicted) |
| Density | 1.401±0.06 g/cm3(Predicted) |
| vapor pressure | 1.84 x 10-3 (Pa 25 °C) |
| Flash point: | >100 °C |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), DMSO (Slightly) |
| form | Solid |
| form | neat |
| Water Solubility | 1.10 mg l-1 (25 °C) |
| color | White to off-white |
| Merck | 13,9587 |
| BRN | 2136521 |
| CAS DataBase Reference | 57018-04-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Phosphorothioic acid, o-(2,6-dichloro-4-methylphenyl) o,o-dimethyl ester(57018-04-9) |
| EPA Substance Registry System | Tolclofos-methyl (57018-04-9) |
Safety information for Tolclofos-methyl
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P272:Contaminated work clothing should not be allowed out of the workplace. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P333+P313:IF SKIN irritation or rash occurs: Get medical advice/attention. |
Computed Descriptors for Tolclofos-methyl
| InChIKey | OBZIQQJJIKNWNO-UHFFFAOYSA-N |
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran




You may like
-
 Tolclofos-methyl CAS 57018-04-9View Details
Tolclofos-methyl CAS 57018-04-9View Details
57018-04-9 -
 Tolclofos-methyl CAS 57018-04-9View Details
Tolclofos-methyl CAS 57018-04-9View Details
57018-04-9 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4