Ticarcillin disodium salt
- CAS NO.:4697-14-7
- Empirical Formula: C15H17N2NaO6S2
- Molecular Weight: 408.42
- MDL number: MFCD07787410
- EINECS: 628-059-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
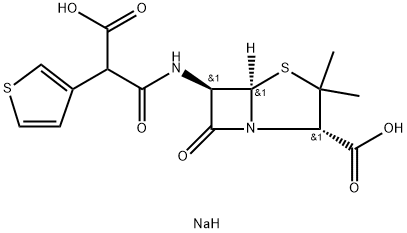
What is Ticarcillin disodium salt?
Description
Ticarcillin is a semisynthetic β-lactam antibiotic. It is active against P. aeruginosa, E. coli, P. mirabilis, P. rettgeri, and K. aerogenes (MICs = 4-125 μg/ml). Topical administration of ticarcillin (2.5 mg per eye) reduces P. aeruginosa colony count in rabbit eye. Formulations containing ticarcillin have been used in the treatment of a variety of bacterial infections.
Chemical properties
Ticarcillin disodium salt is White Solid
Originator
Ticar,Beecham,US,1976
The Uses of Ticarcillin disodium salt
The disodium salt of Ticarcillin, a carboxypenicillin belonging to the beta-lactam class of antibiotics. Ticarcillin is an injectable antibiotic used in the treatment of infections caused by gram-nega tive bacteria, particularly Pseudomonas aeruginosa.
The Uses of Ticarcillin disodium salt
coccidiostat
The Uses of Ticarcillin disodium salt
The disodium salt of Ticarcillin, a carboxypenicillin belonging to the beta-lactam class of antibiotics. Ticarcillin is an injectable antibiotic used in the treatment of infections caused by gram-negative bacteria, particularly Pseudomonas aeruginosa.
What are the applications of Application
Ticarcillin disodium salt is a β-Lactam antibiotic
What are the applications of Application
Ticarcillin/Clavulanate (15/1) is a biochemical that inhibits the cross-linking of peptidoglycan during cell wall synthesis
Definition
ChEBI: Ticarcillin disodium is an organic sodium salt. It contains a ticarcillin(2-).
Manufacturing Process
A mixture of monobenzyl-3-thienylmalonate (1.38 g, 5 mmol) and thionyl
chloride (2.5 ml) was warmed at 50°C to 55°C for 1 hour, then at 60°C to
65°C for 10 minutes. The excess of thionyl chloride was removed in vacuo at
not more than 30°C, the last traces being removed by codistillation with dry
benzene (1 ml) under high vacuum, leaving monobenzyl3-thienylmalonyl
chloride as a yellow oil.
The acid chloride obtained as described above was dissolved in dry acetone
(10 ml) and added in a steady stream to a stirred solution of 6-
aminopenicillanic acid (1.08 g, 5 mmol) in a mixture of N sodium bicarbonate
(15 ml) and acetone (5 ml). After the initial reaction the reaction mixture was
stirred at room temperature for 45 minutes, then washed with ether (3 x 25
ml). Acidification of the aqueous solution with N hydrochloric acid (11 ml) to
pH 2 and extraction with ether (3 x 15 ml) gave an ethereal extract which
was decolorized with a mixture of activated charcoal and magnesium sulfate
for 5 minutes.
The resulting pale yellow ethereal solution was shaken with sufficient N
sodium bicarbonate (4 ml) to give an aqueous extract of pH 7 to 7.5. This
extract was concentrated to syrup at low temperature and pressure, then
isopropanol was added with stirring until the mixture contained about 10%
water.
Crystallization was initiated, and completed at about 0°C overnight, to give
the sodium salt of α-(benzyloxycarbonyl)-3-thienylmethylpenicillin as white
crystals in 50% weight yield. This product was estimated by colorimetric assay
with hydroxylamine to contain 91% of the anhydrous sodium salt.
A solution of the sodium salt of α-(benzyloxycarbonyl)-3-
thienylmethylpenicillin (2.13 g, 4.3 mmol) in water (30 ml) was added to a
suspension of 5% palladium on calcium carbonate (10.65 g) in water (32 ml)
which had been prehydrogenated for 1 hour.
The mixture was then hydrogenated at just above atmospheric pressure for 1
1/2 hours and filtered through a Dicalite bed. The clear filtrate was
evaporated at low temperature and pressure, and the residue dried in vacuo
over phosphorus pentoxide, to give 1.64 g of the salt of α-(3-
thienyl)methylpenicillin as a white solid.
Colorimetric assay with hydroxylamine showed this salt to contain 94% of the anhydrous penicillin. Paper chromatography showed complete reduction of the
benzyl group.
Therapeutic Function
Antibiotic
General Description
Ticarcillin was synthesized by Beecham Research Laboratories in 1971. The phenyl residue of carbenicillin was replaced by the 3-thienyl moiety. Ticarcillin shows almost the same activity as carbenicillin against gram-positive bacteria and a twofold higher activity against gram-negative bacteria. Its in vivo activity against Pseudomonas aeruginosa infections in mice is fourfold higher than that of sulbenicillin. Ticarcillin is used to treat sepsis, urinary tract infections, and serious Pseudomonas aeruginosa, Escherichia coli, Proteus, and Enterobacter infections in leukemic and other cancer patients.
Properties of Ticarcillin disodium salt
| Melting point: | >173°C (dec.) |
| storage temp. | 2-8°C |
| solubility | H2O: soluble50mg/mL |
| form | powder |
| color | white to light yellow |
| Merck | 13,9505 |
| Stability: | Moisture sensitive |
| CAS DataBase Reference | 4697-14-7(CAS DataBase Reference) |
Safety information for Ticarcillin disodium salt
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H334:Sensitisation, respiratory H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ticarcillin disodium salt
| InChIKey | ZBBCUBMBMZNEME-QBGWIPKPSA-L |
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 Ticarcillin Disodium Salt extrapure CAS 4697-14-7View Details
Ticarcillin Disodium Salt extrapure CAS 4697-14-7View Details
4697-14-7 -
 Ticarcillin disodium salt ≥85% CAS 4697-14-7View Details
Ticarcillin disodium salt ≥85% CAS 4697-14-7View Details
4697-14-7 -
 Ticarcillin disodium salt CAS 4697-14-7View Details
Ticarcillin disodium salt CAS 4697-14-7View Details
4697-14-7 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1