Ethylenediaminetetraacetic acid disodium salt
Synonym(s):EDTA disodium salt;Ethylenediaminetetraacetic acid disodium salt dihydrate;Disodium ethylenediaminetetraacetate dihydrate;Edathamil;Edetate disodium salt dihydrate
- CAS NO.:139-33-3
- Empirical Formula: C10H14N2Na2O8
- Molecular Weight: 336.21
- MDL number: MFCD00070672
- EINECS: 205-358-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:11
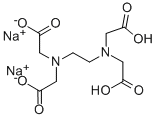
What is Ethylenediaminetetraacetic acid disodium salt?
Chemical properties
Disodium edetate occurs as a white crystalline, odorless powder with a slightly acidic taste.
Originator
Endrate Disodium,Bersworth,US,1959
The Uses of Ethylenediaminetetraacetic acid disodium salt
Ethylenediaminetetraacetic acid disodium salt is widely used in textile industry. Usually applied to dissolve limescale. It is applied in textile industry, pulp and paper industry and also in chelation therapy. In cosmetics, it acts as a sequestering agent. It acts as a corrosion inhibitor to carbon steel in the industries. It also acts as a food additive.
The Uses of Ethylenediaminetetraacetic acid disodium salt
Chelating agent (metal); pharmaceutic aid (chelating agent).
The Uses of Ethylenediaminetetraacetic acid disodium salt
disodium EDTA is a preservative used in concentrations of 0.1 to 0.5 percent.
The Uses of Ethylenediaminetetraacetic acid disodium salt
Disodium Dihydrogen EDTA is a sequestrant and chelating agent whose complete name is disodium ethylenediamine tetraacetate. it is a nonhygroscopic powder that is colorless, odorless, and tasteless at recommended use levels. A 1% solution has a ph of 4.3–4.7. It is used to control the reaction of trace metals to include calcium and magnesium with other organic and inorganic components in food to prevent deterioration of color, texture, and development of precipitates and to prevent oxidation. Its function is comparable to that of disodium calcium edta.
Indications
Edetate disodium is indicated for emergency treatment of hypercalcemia and digitalis toxicity associated ventricular arrhythmias.
Background
Edetate disodium anhydrous is a polyvalent chelating agent used to treat hypercalcemia and digitalis toxicity associated ventricular arrhythmias.
Definition
ChEBI: An organic sodium salt that is the anhydrous form of the disodium salt of ethylenediaminetetraacetic acid (EDTA).
Production Methods
Disodium edetate may be prepared by the reaction of edetic acid and sodium hydroxide.
What are the applications of Application
Ethylenediaminetetraacetic acid disodium salt dihydrate has been in seed germination trials of plant species and in protein extraction from Moss, Physcomitrella paten. It has also been used in lysis and vacuole buffer for the isolation of vacuoles from Petunia petals.
Chelator of divalent cations. Inhibits enzymes, such as metalloproteases, that require divalent cations for activity.
Manufacturing Process
10 mols of ethylene diamine as a 30% aqueous solution and 4 mols of solid
caustic soda are placed in a steam heated kettle supplied with an agitator. 8
mols of sodium cyanide as a concentrated water solution (about 30%) are
added and the solution heated to 60°C. About a 10 inch vacuum is applied to
bring the liquid to incipient boiling. Formaldehyde (7.5 mols of 37% to 40%
aqueous solution) is slowly added, the temperature being held at 60°C, and
the solution vigorously stirred. Then, when the evolution of ammonia has
substantially stopped, an additional 8 mols of sodium cyanide, followed by 8
mols of formaldehyde are added as before. This is continued until 40 mols of
cyanide and 40 mols of formaldehyde have been added. Then at the end
about 2 mols more of formaldehyde are added, making 42 mols in all, to
remove any last traces of cyanide. About 8 to 10 hours are required to
complete the reaction. The resulting product, referred to herein as the crude
reaction product, is essentially an aqueous solution of the sodium salt of
ethylene diamine tetracetic acid.
To 1,000 g of the crude reaction product are added 264 g of ethylene diamine
tetracetic acid. The mixture is preferably heated to incipient boiling to increase
the rate of reaction, and then the mixture is allowed to cool and crystallize.
The crystals formed are filtered off, washed with the smallest possible amount
of ice water, and dried to a constant weight, which is 452 g. A representative
sample of the product so prepared showed, upon analysis, 13.26% sodium
against a theoretical of 13.70% for the disodium salt. The dialkali salt has a
pH of about 5.3 and behaves like a weak acid, displacing CO2 from carbonates
and reacting with metals to form hydrogen. It is a white crystalline solid.
brand name
Endrate (Hospira); Sodium Versenate (3M Pharmaceuticals).
Therapeutic Function
Pharmaceutic aid (chelating agent)
General Description
Ethylenediamine tetraacetate (EDTA) is a calcium ion chelator, that has a low molecular mass of 292.24 Da.
Flammability and Explosibility
Non flammable
Pharmaceutical Applications
Disodium edetate is used as a chelating agent in a wide range of
pharmaceutical preparations, including mouthwashes, ophthalmic
preparations, and topical preparations, typically at concentrations
between 0.005 and 0.1% w/v.
Disodium edetate forms stable water-soluble complexes (chelates)
with alkaline earth and heavy-metal ions. The chelated form
has few of the properties of the free ion, and for this reason
chelating agents are often described as ‘removing’ ions from
solution, a process known as sequestering. The stability of the
metal–edetate complex is dependent on the metal ion involved and
the pH.
Disodium edetate is also used as a water softener as it will chelate
calcium and magnesium ions present in hard water. It is also used
therapeutically as an anticoagulant as it will chelate calcium and
prevent the coagulation of blood in vitro. Concentrations of 0.1%
w/v are used in small volumes for hematological testing and 0.3%
w/v in transfusions.
Biochem/physiol Actions
Ethylenediaminetetraacetic acid disodium salt has the ability to block the binding of vasoactive intestinal peptide to macrophage membranes. It is mainly used in the purification of protein, to remove divalent cations and also to prevent protease activity.
Pharmacokinetics
Edetate disodium anhydrous is a polyvalent ion chelator that reduces blood concentrations of calcium or digitalis. It has a long duration of action as patients are generally given 1 daily dose. The therapeutic index is wide, as high doses are generally well tolerated. Patients should be counselled regarding the risk of postural hypotension, effects of myocardial contractility, hypokalemia, hypomagnesemia, and hypoglycemia.
Safety Profile
Poison by intraperitoneal and intravenous routes. Moderately toxic by ingestion. Experimental teratogenic and reproductive effects. Mutation data reported. The calcium disodium salt of EDTA is used as a chelating agent in treating lead poisoning. When heated to decomposition it emits toxic fumes of NOx and NasO.
Safety
Disodium edetate is used widely in topical, oral, and parenteral
pharmaceutical formulations; it is used extensively in cosmetic and
food products. Disodium edetate and edetate calcium disodium are
used in a greater number and variety of pharmaceutical formulations
than is edetic acid. Both disodium edetate and edetate calcium
disodium are poorly absorbed from the gastrointestinal tract and
are associated with few adverse effects when used as excipients in
pharmaceutical formulations.
Disodium edetate, trisodium edetate, and edetic acid readily
chelate calcium and can, in large doses, cause calcium depletion
(hypocalcemia) if used over an extended period of time, or if
administered too rapidly by intravenous infusion. If used in
preparations for the mouth, they can also leach calcium from the
teeth. However, edetate calcium disodium does not chelate calcium.
Disodium edetate should be used with caution in patients with
renal impairment, tuberculosis, and impaired cardiac function.
Although disodium edetate is generally considered safe, there
have been reports of disodium edetate toxicity in patients receiving
chelation therapy.
Nasal formulations containing benzalkonium chloride and
disodium edetate, both known to be local irritants, were shown to
produce an inflammatory reaction, and microscopic examination
showed an extended infiltration of the mucosa by eosinophils, and
pronounced atrophy and disorganization of the epithelium,
although these effects were subsequently shown to be reversible.
The WHO has set an estimated acceptable daily intake for
disodium EDTA in foodstuffs of up to 2.5 mg/kg body-weight
LD50 (mouse, IP): 0.26 g/kg
LD50 (mouse, IV): 0.056 g/kg
LD50 (mouse, OP): 2.05 g/kg
LD50 (rabbit, IV): 0.047 g/kg
LD50 (rabbit, OP): 2.3 g/kg
LD50 (rat, OP): 2.0 g/kg
Veterinary Drugs and Treatments
Edetate Disodium (Sodium EDTA) is a chelating agent that is also used to stop the melting effect of collagenases and proteases on the cornea. EDTA is useful in halting melting through inhibition of matrix metalloproteinases, but is not felt to be useful for melting caused by infectious agents. As the effect of EDTA on metalloproteinases is reversible, it must be administered several times daily to be effective.
Metabolism
Edetate is almost completely unmetabolized in vivo.
Toxicity
Patients experiencing an overdose may present with calcium deficiency. Treat overdose with symptomatic and supportive treatment, which may include intravenous calcium gluconate.
Storage
Edetate salts are more stable than edetic acid (see also Edetic acid).
However, disodium edetate dihydrate loses water of crystallization
when heated to 120°C. Aqueous solutions of disodium edetate may
be sterilized by autoclaving, and should be stored in an alkali-free
container.
Disodium edetate is hygroscopic and is unstable when exposed
to moisture. It should be stored in a well-closed container in a cool,
dry place.
Incompatibilities
Disodium edetate behaves as a weak acid, displacing carbon dioxide from carbonates and reacting with metals to form hydrogen. It is incompatible with strong oxidizing agents, strong bases, metal ions, and metal alloys.
Regulatory Status
GRAS listed. Included in the FDA Inactive Ingredients Database (inhalations; injections; ophthalmic preparations; oral capsules, solutions, suspensions, syrups, and tablets; rectal topical, and vaginal preparations). Included in nonparenteral and parenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Properties of Ethylenediaminetetraacetic acid disodium salt
| Melting point: | 248 °C (dec.)(lit.) |
| Boiling point: | >100 °C |
| Density | 1.01 g/mL at 25 °C |
| vapor pressure | 0Pa at 25℃ |
| storage temp. | 2-8°C |
| solubility | H2O: clear, colorless |
| form | solution |
| color | ≤5 (0.5 M)(APHA) |
| Odor | at 100.00?%. odorless |
| Water Solubility | Miscible with water. |
| BRN | 3822669 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 139-33-3(CAS DataBase Reference) |
| EPA Substance Registry System | Ethylenediaminetetraacetic acid disodium salt (139-33-3) |
Safety information for Ethylenediaminetetraacetic acid disodium salt
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H303:Acute toxicity,oral H332:Acute toxicity,inhalation H372:Specific target organ toxicity, repeated exposure H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P273:Avoid release to the environment. P312:Call a POISON CENTER or doctor/physician if you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for Ethylenediaminetetraacetic acid disodium salt
Ethylenediaminetetraacetic acid disodium salt manufacturer
Ozone Enterprise
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 EDTA Di Sodium 98%View Details
EDTA Di Sodium 98%View Details -
 EDTA DI SODIUM 99%View Details
EDTA DI SODIUM 99%View Details -
 EDTA - DISODIUM 98%View Details
EDTA - DISODIUM 98%View Details -
 DISODIUM EDTA 99%View Details
DISODIUM EDTA 99%View Details -
 EDTA disodium 99%View Details
EDTA disodium 99%View Details -
 EDTA disodium salt 99%View Details
EDTA disodium salt 99%View Details -
 Edetate disodium dihydrate 98%View Details
Edetate disodium dihydrate 98%View Details -
 Disodium EDTA, For IndustrialView Details
Disodium EDTA, For IndustrialView Details
105-99-7
