thiocyanate
- CAS NO.:302-04-5
- Empirical Formula: CHNS
- Molecular Weight: 58.08
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
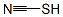
What is thiocyanate?
The Uses of thiocyanate
Thiocyanate is one of the most important spectrophotometric reagents. The availability of the reagent and the simplicity of thiocyanate methods are responsible for its great popularity in analytical laboratories. Thiocyanate is principally used for determination of Fe(III), Mo, W, Nb, Re, Co, U, and Ti.
The determination of metals by thiocyanate is carried out in aqueous or aqueous-acetone media, or after extraction with oxygen-containing solvents. The extractability of metal complexes depends on the acidity of the medium, the concentration of thiocyanate, and the organic solvent. The more acidic is the aqueous phase, and the higher the thiocyanate concentration, the more thiocyanic acid (HSCN) is also extracted by the organic phase.
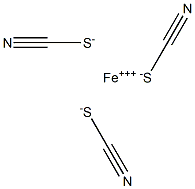
Stepwise formation of thiocyanate complexes gives cationic (e.g., FeSCN2+), neutral [e.g., Fe(SCN)3], and anionic [e.g., Fe(SCN)4-] species. The last is formed at high thiocyanate concentrations. With organic bases such as pyridine, tributylamine, and diantipyrylmethane, anionic thiocyanate complexes form ion-pairs which can be extracted into chloroform and other inert solvents.
Increased selectivity in the determination of metals by thiocyanate is obtained by the choice of acidity, thiocyanate concentration, masking agent, and metal oxidation state. For example, the presence of a reducing agent is necessary for colour reactions with Mo, W, and Re. The reducing medium precludes the colour reaction of thiocyanate with iron.
Thiocyanate methods vary widely in sensitivity. The methods for determining Te, Fe(III), and Nb are highly sensitive, whereas those for U and Co are less sensitive.
The colour stability of some thiocyanate systems is low (e.g., that with iron). This is connected with either the reducing properties of the thiocyanate or the slow polymerization of thiocyanic acid in acid solutions, which causes yellowing. Solvents miscible with water increase the colour intensity of thiocyanate complexes in aqueous solutions. This is apparently owing to the lowered dielectric constants of the media, which inhibit dissociation of the complexes.
Anionic thiocyanate complexes are extractable as ion-association species with basic dyes.
The Uses of thiocyanate
Fumigants.
Definition
ChEBI: A pseudohalide anion obtained by deprotonation of the thiol group of thiocyanic acid.
Definition
thiocyanate: A salt or ester of thiocyanicacid.
Hazard
Rapid-acting poisons, thyrotoxic.
Properties of thiocyanate
| Melting point: | 168-169 °C |
| storage temp. | -20°C |
| EPA Substance Registry System | Thiocyanate (302-04-5) |
Safety information for thiocyanate
Computed Descriptors for thiocyanate
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

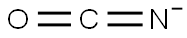
You may like
-
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Potassium Hydroxide 90%View Details
Potassium Hydroxide 90%View Details
1310-58-3 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
