Ferrous chloride tetrahydrate
Synonym(s):Ferrous chloride tetrahydrate;Iron dichloride;Iron(II) chloride tetrahydrate
- CAS NO.:13478-10-9
- Empirical Formula: Cl2FeH2O
- Molecular Weight: 144.76
- MDL number: MFCD00149709
- EINECS: 603-870-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
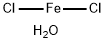
What is Ferrous chloride tetrahydrate?
Chemical properties
light green or blue-green crystals
The Uses of Ferrous chloride tetrahydrate
Iron(II) chloride tetrahydrate is used as a reducing agent in metallurgy, in pharmaceutical preparations, as a mordant in dyeing and in sewage treatment. Ferrous chloride has a kind of niche application. During the laboratory synthesis of iron complexes, ferrous chloride acts as a reducing flocculating agent in waste water treatment, particularly for wastes containing chromate. It is the precursor to hydrated iron(III) oxides that are magnetic pigments. Ferrous chloride is employed as a reducing agent in many organic synthesis reactions.
The Uses of Ferrous chloride tetrahydrate
A variety of organically templated iron phosphates, of interest because of their rich crystal chemistry and their ability to absorb large molecules in their micropores,were prepared using this compound.
Definition
ChEBI: A hydrate that is the tetrahydrate form of iron dichloride.
General Description
Iron(II) chloride tetrahydrate is a hydrated metallic halide. Crystal structure studies suggest that it crystallizes in monoclinic system having space group P21/c.
Purification Methods
A 550mL round-bottomed Pyrex flask is connected, via a glass tube fitted with a medium porosity sintered-glass disc, to a similar flask. To 240g of FeCl2.4H2O in the first flask is added conductivity water (200mL), 38% HCl (10mL), and pure electrolytic iron (8-10g). A stream of purified N2 gas is passed through the assembly, escaping through a mercury trap. The salt is dissolved by heating which is continued until complete reduction has occurred. By inverting the apparatus and filtering (under N2 pressure) through the sintered glass disc, unreacted iron is removed. After cooling and crystallisation, the unit is again inverted, and the crystals of ferrous chloride are filtered free from mother liquor by applied N2 pressure. Partial drying by overnight evacuation at room temperature gives a mixed hydrate which, on further evacuation on a water bath at 80o, loses water of hydration and absorbed HCl (with vigorous effervescence) to give a white powder of FeCl2.2H2O (see below). [Gayer & Wootner J Am Chem Soc 78 3944 1956, (2H2O) Gayer & Woontner Inorg Synth V 179 1957.]
Properties of Ferrous chloride tetrahydrate
| Melting point: | 105 °C |
| Boiling point: | 1026 °C |
| Density | 1.93 |
| vapor pressure | 10 mm Hg ( 693 °C) |
| storage temp. | Store at +15°C to +25°C. |
| solubility | 1600g/l |
| form | Solid |
| color | Yellow to green |
| Specific Gravity | 1.93 |
| PH | 2.5 (100g/l, H2O, 20℃) |
| Water Solubility | soluble |
| Sensitive | Air Sensitive |
| Merck | 14,4043 |
| Exposure limits | ACGIH: TWA 1 mg/m3 NIOSH: TWA 1 mg/m3 |
| Stability: | Air, moisture and light-sensitive. Incompatible with strong oxidizing agents, most common metals. |
| CAS DataBase Reference | 13478-10-9(CAS DataBase Reference) |
Safety information for Ferrous chloride tetrahydrate
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P501:Dispose of contents/container to..… |
Computed Descriptors for Ferrous chloride tetrahydrate
| InChIKey | WSSMOXHYUFMBLS-UHFFFAOYSA-L |
Ferrous chloride tetrahydrate manufacturer
ALPHA CHEMIKA
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 13478-10-9 Ferrous Chloride Tetrahydrate 98%View Details
13478-10-9 Ferrous Chloride Tetrahydrate 98%View Details
13478-10-9 -
 FERROUS CHLORIDE TETRAHYDRATE 99%View Details
FERROUS CHLORIDE TETRAHYDRATE 99%View Details
13478-10-9 -
 Ferrous Chloride Tetrahydrate extrapure CAS 13478-10-9View Details
Ferrous Chloride Tetrahydrate extrapure CAS 13478-10-9View Details
13478-10-9 -
 Ferrous chloride, hydrate CAS 13478-10-9View Details
Ferrous chloride, hydrate CAS 13478-10-9View Details
13478-10-9 -
 Ferrous chloride, hydrate, GR 99%+ CAS 13478-10-9View Details
Ferrous chloride, hydrate, GR 99%+ CAS 13478-10-9View Details
13478-10-9 -
 Iron(II) chloride tetrahydrate CAS 13478-10-9View Details
Iron(II) chloride tetrahydrate CAS 13478-10-9View Details
13478-10-9 -
 FERROUS CHLORIDE HYDRATED Extra Pure CAS 13478-10-9View Details
FERROUS CHLORIDE HYDRATED Extra Pure CAS 13478-10-9View Details
13478-10-9 -
 Iron(ii) chloride tetrahydrate 99% CAS 13478-10-9View Details
Iron(ii) chloride tetrahydrate 99% CAS 13478-10-9View Details
13478-10-9
