Thiamine chloride
- CAS NO.:59-43-8
- Empirical Formula: C12H17ClN4OS
- Molecular Weight: 300.81
- MDL number: MFCD00044586
- EINECS: 200-425-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
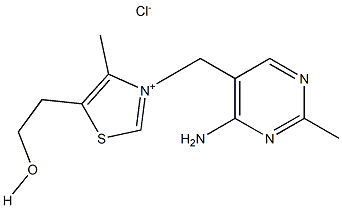
What is Thiamine chloride?
Originator
Thiamine chloride,Sopharma
The Uses of Thiamine chloride
Vitamin B1 is used in method for cultivating tremella strain. Also, it is protein compound liquid comprising collagen and Rhodiola rosea extract or preparing injection to remove skin wrinkle.
Definition
ChEBI: Thiamine(1+) chloride is a vitamin B1 and an organic chloride salt. It contains a thiamine(1+).
Manufacturing Process
2 Methods of preparation of thiamine:
1. 3-Ethoxypropionitrile reacted with diethoxymethoxy-ethane and 3-ethoxy-
2-methoxymethylenpropionitrile was obtained.
Then to the 3-ethoxy-2-methoxymethylenpropionitrile acetamidine was added
and reaction mixture was stirred to give 4-amino-5-ethoxy-methyl-2-
methylpyrimidine.
The 4-amino-5-ethoxy-methyl-2-methylpyrimidine was treated by hydrobromic
acid to afford 4-amino-5-bromomethyl-2-methylpyrimidine hydrobromide.
The 4-amino-5-bromomethyl-2-methylpyrimidine hydrobromide reacted with
5-(2-hydroxyethyl)-4-methylthiazole in the presence of hydrobromic acid and
as the result thiaminbromide was produced. For changing of the thiaminbromide to the thiamincloride the thiaminbromide was treated by AgCl.
2. To diethoxymethoxy-ethane malononitrile was added and ethoxymethylenmalononitrile
was obtained. The ethoxymethylen-malononitrile reacted with
acetamidine as a result 4-amino-5-cyano-2-methylpyrimidine was produced,
which was reduced by H2/Raney-Ni to 4-amino-5-aminomethyl-2-
methylpyrimidine.
To the 4-amino-5-aminomethyl-2-methylpyrimidine 1-acetoxy-3-chloropentan-
4-one was added in the presence CS2 and NH3, and reaction mixture was
stirred, then to this mixture hydrochloric acid was added and thiamin (base)
was obtained.
To thiamin (base) H2O2 and hydrocloricum acid are added, in the result
reaction the thiamine chloride was obtained.
Therapeutic Function
Enzyme cofactor vitamin, Antineuritic
General Description
Thiamine, the preferred name for vitamin B1, holds a prominent place in the history of vitamin discovery because beriberi, the disease resulting from insufficient thiamine intake, was one of the earliest recognized deficiency diseases.
Biological Activity
Some earlier designations for this substance included aneurin, antineuritic factor, antiberiberi factor, and oryzamin. Thiamine is metabolically active as thiamine pyrophosphate (TPP).
TPP functions as a coenzyme which participates in decarboxylation of α-keto acids. Dehydrogenation and decarboxylation must precede the formation of “active acetate” in the initial reaction of the TCA cycle (citric acid cycle):

This reaction is a good example of the interrelationship of vitamin B coenzymes. Four vitamin coenzymes are necessary for this one reaction: (1) thiamine (in TPP) for decarboxylation; (2) nicotinic acid in nicotinamide adenine dinucleotide (NAD); (3) riboflavin in flavin adenine dinucleotide (FAD); and (4) pantothenic acid in coenzyme A (CoA) for activation of the acetate fragment.
Clinical Use
Thiamine chloride, as the base or as the hydrochloride salt, is indicatedin the treatment or prophylaxis of known or suspected thiaminedeficiencies. Severe thiamine deficiency is calledberiberi, which is very rare in developed countries. The mostlikely cause of thiamine deficiency in the United States is theresult of chronic alcoholism, which leads to multiple vitamindeficiencies as a result of poor dietary intake. The major organsaffected are the nervous system (dry beriberi), whichmanifests as neurological damage, the cardiovascular system(wet beriberi), which manifests as heart failure and edema, andthe gastrointestinal tract. Thiamine administration reverses thegastrointestinal, cardiovascular, and neurological symptoms;however, if the deficiency has been severe or of prolonged duration, the neurological damage may be permanent.
Safety Profile
Poison by subcutaneous and intravenous routes. An experimental teratogen. Experimental reproductive effects. When heated to decomposition it emits very toxic fumes of NOx, SOx, and Cl-.
Properties of Thiamine chloride
| Melting point: | 248 °C (decomp) |
| Density | 1.3175 (rough estimate) |
| refractive index | 1.5630 (estimate) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | DMSO : 6 mg/mL (19.95 mM) |
| form | Solid |
| color | White to off-white |
| InChI | InChI=1S/C12H17N4OS.ClH/c1-8-11(3-4-17)18-7-16(8)6-10-5-14-9(2)15-12(10)13;/h5,7,17H,3-4,6H2,1-2H3,(H2,13,14,15);1H/q+1;/p-1 |
| CAS DataBase Reference | 59-43-8(CAS DataBase Reference) |
| EPA Substance Registry System | Thiamine (59-43-8) |
Safety information for Thiamine chloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H312:Acute toxicity,dermal H332:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P330:Rinse mouth. P363:Wash contaminated clothing before reuse. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P501:Dispose of contents/container to..… |
Computed Descriptors for Thiamine chloride
| InChIKey | MYVIATVLJGTBFV-UHFFFAOYSA-M |
| SMILES | O([H])CCC1=C(C)[N+](=CS1)CC1C=NC(=NC=1N)C.[Cl-] |
Thiamine chloride manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 59-43-8 Thiamine 98%View Details
59-43-8 Thiamine 98%View Details
59-43-8 -
 59-43-8 99%View Details
59-43-8 99%View Details
59-43-8 -
 Thiamine 98%View Details
Thiamine 98%View Details
59-43-8 -
 Vitamin B1 98.00% CAS 59-43-8View Details
Vitamin B1 98.00% CAS 59-43-8View Details
59-43-8 -
 Medico Vitamin B1, TabletsView Details
Medico Vitamin B1, TabletsView Details
59-43-8 -
 Vitamin B1 4Percent Extract PowderView Details
Vitamin B1 4Percent Extract PowderView Details
59-43-8 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
