Diphenylphosphine
Synonym(s):HPPh2;Ph2PH
- CAS NO.:829-85-6
- Empirical Formula: C12H11P
- Molecular Weight: 186.19
- MDL number: MFCD00003040
- EINECS: 212-591-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
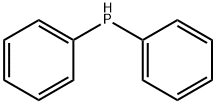
What is Diphenylphosphine?
Chemical properties
Diphenylphosphine is an organophosphorus compound commonly used in laboratories. It is a clear colorless to slightly yellow liquid with unpleasant odor, irritating, easily oxidized in air and spontaneously combusts, sensitive to air and light, and needs to be protected by nitrogen. It can be used as a precursor for the synthesis of a variety of organophosphine ligands. These ligands, in turn, are used in homogeneous catalysis for many applications including: asymmetric hydrogenation, coupling chemistry, ethylene oligomerization, hydroformylation, hydration of nitriles, and polymerization of alkenes.
The Uses of Diphenylphosphine
Diphenylphosphine is used in the synthesis of aminophosphines for application as catalysts. It is also used in the preparation of chiral palladacycles with N-heterocyclic carbene ligands as catalysts.
The Uses of Diphenylphosphine
suzuki reaction
The Uses of Diphenylphosphine
Diphenylphosphine acts as an intermediate in the preparation of diphenylphosphide derivatives, phosphonium salts, phosphine ligands and Wittig-Horner reagents. The presence of hydrogen atom bonded to phosphorus undergoes Michael-like addition to activated alkenes. It is involved in the preparation of 1,2-bis(diphenylphosphino)ethane and (phenyl-(phenylmethyl)phosphoryl)benzene. Further, it is used in the synthesis of aminophosphines and chiral palladacycles with N-heterocyclic carbene ligands as catalysts.
Preparation
Diphenylphosphine can be prepared from triphenylphosphine by reduction to lithium diphenylphosphide, which can be protonated to give the title compound:
PPh3 + 2 Li → LiPPh2 + LiPh
LiPPh2 + H2O → Ph2PH + LiOH
Properties of Diphenylphosphine
| Melting point: | -14.5 °C |
| Boiling point: | 280 °C(lit.) |
| Density | 1.07 g/mL at 25 °C(lit.) |
| vapor pressure | 2 mm Hg ( 110 °C) |
| refractive index | n |
| Flash point: | -18°C (Hexane) |
| storage temp. | 2-8°C |
| solubility | Chloroform |
| form | liquid |
| pka | diphenylphosphine is weakly basic. The pKa of the protonated derivative is 0.03. |
| Specific Gravity | 0.68 |
| color | colorless |
| Water Solubility | Miscible with ethanol, ether, benzene, concentrated hydrochloric acid. Immiscible with water. |
| Sensitive | Air & Moisture Sensitive |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| BRN | 742504 |
| CAS DataBase Reference | 829-85-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Phosphine, diphenyl-(829-85-6) |
Safety information for Diphenylphosphine
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H250:Pyrophoric liquids; Pyrorophoric solids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P222:Do not allow contact with air. P231+P232:Handle under inert gas. Protect from moisture. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P370+P378:In case of fire: Use … for extinction. |
Computed Descriptors for Diphenylphosphine
| InChIKey | GPAYUJZHTULNBE-UHFFFAOYSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Diphenylphosphine, 98% CAS 829-85-6View Details
Diphenylphosphine, 98% CAS 829-85-6View Details
829-85-6 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
