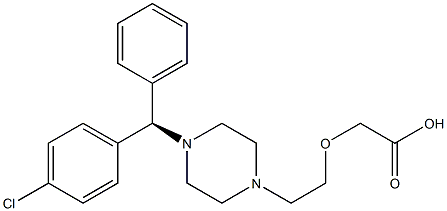
Cetirizine
- CAS NO.:83881-51-0
- Empirical Formula: C21H25ClN2O3
- Molecular Weight: 388.89
- MDL number: MFCD00800721
- EINECS: 251-228-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52

What is Cetirizine?
Absorption
Cetirizine was rapidly absorbed with a time to maximum concentration (Tmax) of about 1 hour after oral administration of tablets or syrup formulation in adult volunteers . Bioavailability was found to be similar between the tablet and syrup dosage forms. When healthy study volunteers were given several doses of cetirizine (10 mg tablets once daily for 10 days), a mean peak plasma concentration (Cmax) of 311 ng/mL was measured .
Effect of food on absorption
Food had no effect on cetirizine exposure (AUC), however, Tmax was delayed by 1.7 hours and Cmax was decreased by 23% in the fed state .
Toxicity
Oral LD50 (rat): 365 mg/kg; Intraperitoneal LDLO (mouse): 138 mg/kg; Oral TDLO (rat): 50 mg/kg; Oral TDLO (mouse): 0.1 mg/kg .
Carcinogenesis and mutagenesis: In a 2-year carcinogenicity study in rats, cetirizine was not shown to be carcinogenic at dietary doses up to 20 mg/kg (approximately 15 times the maximum recommended daily oral dose in adults). In a 2-year carcinogenicity study in mice, cetirizine administration lead to an incidence of benign liver tumors in males at a dietary dose of 16 mg/kg (approximately 6 times the maximum recommended daily oral dose in adults). The clinical significance of these findings during long-term use of cetirizine is unknown at this time .
Cetirizine was not mutagenic in the Ames test, and not clastogenic in the human lymphocyte assay, the mouse lymphoma assay, and in vivo micronucleus test in rats .
Impairment of fertility
In a fertility and reproduction study in mice, cetirizine did not negatively impact fertility at an oral dose of 64 mg/kg (approximately 25 times the maximum recommended daily oral dose in adults) .
Pregnancy Category B:
In mice, rats, and rabbits, cetirizine was not teratogenic at oral doses up to 96, 225, and 135 mg/kg, respectively (approximately 40, 180 and 220 times the maximum recommended daily oral dose in adults). There are no adequate and well-controlled studies in pregnant women. Because animal studies are not always predictive of human response, cetirizine should be used in pregnancy only if clearly needed .
Use in breastfeeding/nursing
Cetirizine has been reported to be excreted in human breast milk. The use of cetirizine in nursing mothers is not recommended .
Chemical properties
white, crystalline powder
The Uses of Cetirizine
Cetirizine is a metabolite of the first-generation H1 antihistamine hydroxyzine. A reduced dosage (5 mg daily) is recommended in patients with chronic renal or hepatic impairment. Sedative side effects increase with dosages of cetirizine >10 mg per day.
Background
Cetirizine, also commonly known as Zyrtec, is an orally active second-generation histamine H1 antagonist proven effective in the treatment of various allergic symptoms, such as sneezing, coughing, nasal congestion, hives, and other symptoms , .
One of the most common uses for this drug is for a condition called allergic rhinitis. The prevalence of allergic rhinitis in the United States is about 15% according to physician diagnoses, and up to 30%, according to self-reported nasal symptoms. Allergic rhinitis is associated with multiple missed or unproductive days at work and school, problems with sleep, and other difficulties with day to day activities for many individuals . Furthermore, some antihistamine agents that are used to treat this condition cause undesirable, sedating effects .
Cetirizine is one of the first second-generation H1 antihistamines (SGAHs) formulated to selectively inhibit the H1 receptor without sedating effects .
Indications
Seasonal Allergic Rhinitis: Indicated for the relief of symptoms associated with seasonal allergic rhinitis caused by allergens such as ragweed, grass and tree pollens in adults and children 2 years of age and above. Symptoms treated effectively include sneezing, rhinorrhea, nasal pruritus, ocular pruritus, tearing, and redness of the eyes .
Perennial allergic rhinitis: This drug is indicated for the relief of symptoms associated with perennial allergic rhinitis due to allergens including dust mites, animal dander, and molds in adults and children 6 months of age and older. Symptoms treated effectively include sneezing, rhinorrhea, postnasal discharge, nasal pruritus, ocular pruritus, and tearing .
Chronic urticaria: Cetirizine is indicated for the treatment of the uncomplicated skin manifestations of chronic idiopathic urticaria in adults and children 6 months of age and older. It markedly reduces the occurrence, severity, and duration of hives and significantly reduces pruritus .
Definition
ChEBI: A member of the class of piperazines that is piperazine in which the hydrogens attached to nitrogen are replaced by a (4-chlorophenyl)(phenyl)methyl and a 2-(carboxymethoxy)ethyl group respectively.
brand name
Zyrtec (Pfizer).
General Description
Cetirizine, (±)-[2-[4-[(4-chlorophenyl)phenylmethyl]- 1-piperazinyl]ethoxy]acetic acid (Zyrtec), is a racemic compound available as a white crystalline powder that is water soluble. Cetirizine is the primary acid metabolite of hydroxyzine, resulting from complete oxidation of the primary alcohol moiety. This compound is zwitterionic and relatively polar and thus does not penetrate or accumulate in the CNS. Before its introduction in the United States, cetirizine was one of the most widely prescribed H1-antihistamines in Europe.
Cetirizine is indicated for the temporary relief of runny nose, sneezing, itching of the nose or throat, and/or itchy, watery eyes caused by hay fever or other upper respiratory allergies. It also relieves itching caused by hives (urticaria), but it will not prevent hives or an allergic skin reaction from occurring.
Biological Activity
cetirizine, a second-generation antihistamine, is a major metabolite of hydroxyzine, and a racemic selective h1 receptor inverse agonist used in the treatment of allergies, hay fever, angioedema, and urticaria. cetirizine crosses the blood-brain barrier only slightly, reducing the sedative side-effect common with older antihistamines. it has also been shown to inhibit eosinophil chemotaxis and ltb4 release. at a dosage of 20 mg, boone et al. found that it inhibited the expression of vcam-1 in patients with atopic dermatitis. the levorotary enantiomer of cetirizine, known as levocetirizine, is the more active form. from wikipedia.
Pharmacokinetics
General effects and respiratory effects
Cetirizine, the active metabolite of the piperazine H1-receptor antagonist hydroxyzine, minimizes or eliminates the symptoms of chronic idiopathic urticaria, perennial allergic rhinitis, seasonal allergic rhinitis, allergic asthma, physical urticaria, and atopic dermatitis.
The clinical efficacy of cetirizine for allergic respiratory diseases has been well established in numerous trials .
Effects on urticaria/anti-inflammatory effects
It has anti-inflammatory properties that may play a role in asthma management . There is evidence that cetirizine improves symptoms of urticaria. Marked clinical inhibition of a wheal and flare response occurs in infants, children as well as adults within 20 minutes of one oral dose and lasts for 24 h . Concomitant use of cetirizine reduces the duration and dose of topical anti-inflammatory formulas used for the treatment of atopic dermatitis .
Clinical Use
Cetirizine, the acid metabolite from oxidation of the primary alcohol of the antihistamine hydroxyzine, is a widely used antihistamine. It has a long duration of action and is highly selective for H1 receptors. No cardiotoxicity has been reported, but some drowsiness occurs.
Metabolism
A mass balance clinical trial comprised of 6 healthy male study volunteers showed that 70% of the administered radioactivity was measured in the urine and 10% in the feces after cetirizine administration. About 50% of the radioactivity was measured in the urine as unchanged cetirizine. Most of the rapid increase in peak plasma radioactivity was related to the parent drug, implying a low level of first pass metabolism. This prevents potential interactions of cetirizine with drugs interacting with hepatic cytochrome enzymes .
Cetirizine is metabolized partially by oxidative O-dealkylation to a metabolite with insignificant antihistaminic activity. The enzyme or enzymes responsible for this step in cetirizine metabolism have not yet been identified .
Properties of Cetirizine
| Melting point: | 110-115°C |
| Boiling point: | 542.1±45.0 °C(Predicted) |
| Density | 1.237±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | insoluble in EtOH; ≥122 mg/mL in H2O; ≥19.44 mg/mL in DMSO |
| form | solid |
| pka | 3.46±0.10(Predicted) |
| color | Off-white to light yellow |
| Water Solubility | 101 mg/L |
| CAS DataBase Reference | 83881-51-0(CAS DataBase Reference) |
| EPA Substance Registry System | Acetic acid, 2-[2-[4-[(4-chlorophenyl)phenylmethyl]-1-piperazinyl]ethoxy]- (83881-51-0) |
Safety information for Cetirizine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Cetirizine
Cetirizine manufacturer
New Products
Tetrabutylammonium hydrogen sulfate 2,2,6-Trimethyl-4H-1,3-dioxin-4-one 4-Piperidinopiperidine tert-Butyl acetoacetate 4-BROMOMETHYLTETRAHYDROPYRAN 3-Hydroxyazetidine hydrochloride Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) 3-Methoxybenzonitrile 4-Methoxybenzonitrile Dibenzoyl Peroxide Titanium Dioxide Chloral PentachlorobenzonitrileRelated products of tetrahydrofuran


You may like
-
 83881-51-0 CETIRIZINE BASE 98%View Details
83881-51-0 CETIRIZINE BASE 98%View Details
83881-51-0 -
 83881-51-0 98%View Details
83881-51-0 98%View Details
83881-51-0 -
 83881-51-0 Cetirizine 98%View Details
83881-51-0 Cetirizine 98%View Details
83881-51-0 -
 83881-51-0 98%View Details
83881-51-0 98%View Details
83881-51-0 -
 Cetirizine 98%View Details
Cetirizine 98%View Details
83881-51-0 -
 Cetirizine 83881-51-0 99%View Details
Cetirizine 83881-51-0 99%View Details
83881-51-0 -
 Cetirizine 98%View Details
Cetirizine 98%View Details
83881-51-0 -
 Cetirizine 98%View Details
Cetirizine 98%View Details
83881-51-0
