Loratadine
Synonym(s):4-(8-Chloro-5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene-1-piperidinecarboxylic acid ethyl ester;Loratadine;Loratidine
- CAS NO.:79794-75-5
- Empirical Formula: C22H23ClN2O2
- Molecular Weight: 382.88
- MDL number: MFCD00672869
- EINECS: 935-907-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-30 18:11:34
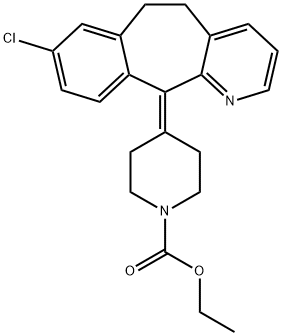
What is Loratadine?
Absorption
Loratadine is rapidly absorbed and achieves peak plasma concentration in 1-2 hours, while it's main metabolite achieves peak plasma concentration in 3-4 hours.
In the rapid dissolve formulation, the pharmacokinetic parameters of loratadine are as follows: Cmax = 2.56 ng/ml, Tmax = 1.14 hrs, AUC = 6.14 ng x hr/ml.
In the conventional formulation, the pharmacokinetic parameters of loratadine are as follows: Cmax = 2.11 ng/ml, Tmax = 1.00 hr, AUC = 4.64 ng x hr/ml.
Toxicity
Second generation antihistamines such as loratadine have very few adverse effects; however, insomnia, headache, fatigue, drowsiness and rash have been reported. Symptoms of loratadine overdose include gastrointestinal side effects, agitation, drowsiness, tachycardia, and headache. It is advised to obtain an ECG in the event of loratadine overdose.
Description
Loratadine is a non-sedating antihistamine indicated for use in allergic rhinitis and chronic urticaria. Its major advantage over other non-sedating antihistamines such as astemizole and terfenadine is its very fast onset of action. Loratadine is claimed not to cross the blood-brain barrier.
Chemical properties
White Crystalline Solid
Originator
Schering Plough (USA)
The Uses of Loratadine
Loratadine, is used as a peripheral histamine H1 receptor agonist. It is also used as an orally active antiallergic agent. It is an important raw material and intermediate used in Organic Synthesis, Pharmaceuticals, Agrochemicals and Dyestuff.
The Uses of Loratadine
A nonsedating-type histamine H1-receptor
The Uses of Loratadine
antinflammatory, analgesic, antipyretic
The Uses of Loratadine
Histamine H1 receptor antagonist; antihistamine.
Background
Loratadine is a second generation antihistamine used to manage symptoms of allergic rhinitis. A lack of sedative and CNS adverse effects make loratadine, along with other second generation antihistamines, preferable over their 1st generation counterparts in many clinical situations.
Indications
Loratadine (Claritin) is a long-acting, potent peripheral H1 blocker with minimal sedative effects. It does not appear to have the same adverse cardiac effects as the other nonsedating H1 antihistamines. It is indicated for allergic rhinitis and chronic urticaria.
Definition
ChEBI: Loratadine is a benzocycloheptapyridine that is 6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridine substituted by a chloro group at position 8 and a 1-(ethoxycarbonyl)piperidin-4-ylidene group at position 11. It is a H1-receptor antagonist commonly employed in the treatment of allergic disorders. It has a role as a geroprotector, a H1-receptor antagonist, an anti-allergic agent and a cholinergic antagonist. It is an ethyl ester, a N-acylpiperidine, a tertiary carboxamide, an organochlorine compound and a benzocycloheptapyridine. It is functionally related to a desloratadine.
Manufacturing Process
Preparation of Loratadine
In a two-liter vessel provided with a thermometer, a reflux condenser and nitrogen atmosphere, dry tetrahydrofuran (343 ml) was placed, and cooled between 0 and -5°C. Titanium tetrachloride (28.5 ml, 49.5 g, 0.255 mol) was slowly added with stirring (17 min.), keeping the temperature in the above indicated range, a yellow suspension being formed. After the addition was finished, stirring was continued for 10 min. Then, zinc dust (34.5 g, 0.524 mol) was added with stirring in approximately 15 min. keeping the temperature in the above cited range, and after addition was finished, stirring was continued at this temperature for 20 min., a blue suspension being formed. Then, pyridine (17 ml, 0.21 mol) was added with stirring, keeping the temperature in the above range, and then, a solution of 8-chloro-5,6- dihydrobenzo[5,6]cyclohepta[1,2-b]pyridin-11-one (30.0 g, 0.123 mol) and ethyl 4-oxopiperidine-1-carboxylate (25.2 g, 0.147 mol) in anhydrous tetrahydrofuran (96 ml) was added in about 20 min., with stirring and keeping the temperature in the above cited range. The, thus obtained, dark brown mixture was stirred for 3 h keeping the temperature in the above cited range, then was allowed to heat to room temperature and kept at this temperature for 2 h and then heated to 40°C for 17 h. The tetrahydrofuran was distilled off
from the reaction mixture to give a black resin that was dissolved in
dichloromethane (300 ml) and acidified by addition of isopropanol/HCl 7.2 N
(97 ml). The mixture was stirred for 10 min, and the phases were separated,
being the aqueous one extracted with dichloromethane (150 ml). The
combined organic phases were washed 6 times with a mixture of water (125
ml) and 35% aqueous HCl (7.5 ml). Then, the organic phase was basified to
pH 7.5-8.0 by addition of 30% aqueous NH3. The mixture was stirred for 10
min and the phases were separated, and then washed 3 times with water
(250 ml). The organic phase was dried with anhydrous sodium sulfate, filtered
and the solvent eliminated in vacuo to give a residue (47.47 g) that was
treated with acetonitrile (97 ml). The solid was filtered and crystallized from
the same solvent to give pure Loratadine, m.p. 132-133°C (18.8 g, 40%
yield).
brand name
Alavert (Wyeth); Claritin (Schering-Plough).
Therapeutic Function
Antihistaminic, Antiallergic
General Description
Loratadine, 4-(8-chloro-5,6-dihydro-11Hbenzo[5,6]-cyclohepta[1,2-b]pyridin-l 1-ylidene-1-carboxylic acid ethyl ester, is a white to off-white powder insoluble in water but very soluble in acetone, alcohols, and chloroform. Loratadine is structurally related to the antihistamines azatadine and cyproheptadine, and to some tricyclic antidepressants. It differs from azatadine, in that a neutral carbamate group has replaced the basic tertiary amino moiety, and a phenyl ring has been substituted with a chlorine atom. Loratadine is a selective peripheral H1-antihistamine with a receptor-binding profile like that of the other members of this series, except that it has more antiserotonergic activity. Thus, it produces no substantial CNS or autonomic side effects or cardiac toxicity.
Loratadine displays potency comparable with that of astemizole and greater than that of terfenadine. Loratadine is indicated for the relief of nasal and nonnasal symptoms of seasonal allergic rhinitis. In the presence of a CYP3A4 inhibitor ketoconazole, loratadine is metabolized to descarboethoxyloratadine predominantly by CYP2D6.
Biological Activity
Peripheral histamine H 1 receptor antagonist (K i = 35 nM); devoid of central effects. Orally active antiallergic agent.
Biochem/physiol Actions
Non-sedating histamine H1-receptor antagonist.
Pharmacokinetics
Like other 2nd generation antihistamines, loratadine is selective for peripheral H1 receptors. Loratadine does not penetrate effectively into the central nervous system and has poor affinity for CNS H1-receptors. These qualities result in a lack of CNS depressant effects such as drowsiness, sedation, and impaired psychomotor function.
Pharmacokinetics
Good oral absorption, rapid and extensive metabolism in the liver, and excreted by urine and feces. After taking the medicine, the effect is fast, and some patients show the effect within 30 minutes. Tmax is 1.5 ~ 2 h, and the elimination half-life is 8 ~ 14 h. The half-life of the active metabolite decarboxymethylethoxyloratadine (DCL) is 17 ~ 24h. The half-life of the elderly and patients with liver disease may be longer. The binding rate of loratadine to plasma protein was 97% ~ 99%, and DCL was 73% ~ 76%. After 24 hours, about 27% of loratadine was excreted from urine, about 40% was eliminated from urine and 42% was excreted from stool after 10 days. With less milk secretion, it is safe to use drugs during lactation.
Clinical Use
Loratadine is related to the first-generation tricyclic antihistamines and to antidepressants. It is nonsedating, and neither it nor its major metabolite, desloratadine (descarboethoxyloratadine), is associated with the potentially cardiotoxic effects reported for terfenadine and astemizole. On chronic dosing, the AUC (plasma concentration–time curve) for the metabolite is greater than that for the parent drug, and its half-life is longer.
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: concentration possibly increased by
erythromycin.
Antifungals: concentration of loratadine possibly
increased by ketoconazole – avoid concomitant use.
Antivirals: concentration possibly increased by
ritonavir.
Metabolism
Loratadine undergoes extensive first pass metabolism in the liver and is primarily metabolized by CYP3A4, CYP2D6, CYP1A1 and CYP2C19. Less involved CYP enzymes include CYP1A2, CYP2B6, CYP2C8, CYP2C9 and CYP3A5. CYP3A4 and CYP2D6 are mainly responsible for metabolizing loratadine to descarboethoxyloratadine. This primary metabolite is 4 times more pharmacologically active than loratadine.
In addition, a study demonstrates that descarboethoxyloratadine is first glucuronidated by UGT2B10, then hydroxylated by CYP2C8 to form 3-hydroxydesloratadine. Further glucuronidation of 3-hydroxydesloratadine facilitates excretion.
Metabolism
Loratadine undergoes an extensive first pass metabolism, mainly by CYP3A4 and CYP2D6. The major metabolite-desloratadine is pharmacologically active and responsible for a large part of the clinical effect. Approximately 40% of the dose is excreted in the urine and 42% in the faeces over a 10 day period and mainly in the form of conjugated metabolites. Approximately 27% of the dose is eliminated in the urine during the first 24 hours. Less than 1% of the active substance is excreted unchanged in the active form, as loratadine or desloratadine.
Storage
Store at RT
Dosage forms
10 mg daily. Patients with liver or renal impairment should be started on a lower dose.
Properties of Loratadine
| Melting point: | 134-136°C |
| Boiling point: | 531.3±50.0 °C(Predicted) |
| Density | 1.261±0.06 g/cm3(Predicted) |
| RTECS | TM6129200 |
| storage temp. | 2-8°C |
| solubility | DMSO: 26 mg/mL, soluble |
| pka | 4.27±0.20(Predicted) |
| form | powder |
| color | white |
| Water Solubility | It is soluble in DMSO (50 mg/ml), ethanol (77 mg/ml at 25°C), water (<1 mg/ml at 25°C), chloroform, and methanol. |
| Merck | 14,5578 |
| Stability: | Stable, but may be heat sensitive - refrigerate. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 79794-75-5(CAS DataBase Reference) |
| EPA Substance Registry System | 1-Piperidinecarboxylic acid, 4-(8-chloro-5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)-, ethyl ester (79794-75-5) |
Safety information for Loratadine
Computed Descriptors for Loratadine
| InChIKey | JCCNYMKQOSZNPW-UHFFFAOYSA-N |
Loratadine manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

![4,8-Dichloro-5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-β]pyridin-11-one (Loratadine Impurity)](https://img.chemicalbook.in/CAS/GIF/133330-60-6.gif)


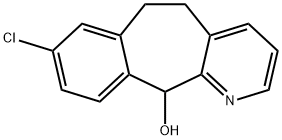
You may like
-
 Loratadine 98%View Details
Loratadine 98%View Details -
 79794-75-5 98%View Details
79794-75-5 98%View Details
79794-75-5 -
 Loratadine 79794-75-5 98%View Details
Loratadine 79794-75-5 98%View Details
79794-75-5 -
 79794-75-5 99%View Details
79794-75-5 99%View Details
79794-75-5 -
 Loratadine 97% CAS 79794-75-5View Details
Loratadine 97% CAS 79794-75-5View Details
79794-75-5 -
 Loratadine CAS 79794-75-5View Details
Loratadine CAS 79794-75-5View Details
79794-75-5 -
 Loratadine Powder APIView Details
Loratadine Powder APIView Details
79794-75-5 -
 Loratadine API PowderView Details
Loratadine API PowderView Details
79794-75-5
