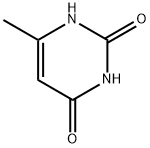
Terbacil
Synonym(s):3-tert-Butyl-5-chloro-6-methyluracil
- CAS NO.:5902-51-2
- Empirical Formula: C9H13ClN2O2
- Molecular Weight: 216.66
- MDL number: MFCD00055344
- EINECS: 227-595-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30

What is Terbacil?
Chemical properties
Colorless crystals. Mp 175C. Soluble in dimethylacetamide and cyclohexanone; partially soluble in xylene and butyl acetate.
The Uses of Terbacil
Terbacil is also absorbed by plant roots and inhibits photosynthesis. It is used for the selective control of many annual and some perennial weeds in apples, citrus, peaches, and sugarcane. Application rates range from 0.5 to 8 kg/ha.
The Uses of Terbacil
Herbicide.
The Uses of Terbacil
Nonselective herbicide used to control herbaceous and woody plants on noncrop land.
Definition
ChEBI: Terbacil is an organohalogen compound and a member of pyrimidines.
General Description
Colorless crystals. Non corrosive. Used as an herbicide.
Air & Water Reactions
Water soluble.
Reactivity Profile
A uracil derivative.
Agricultural Uses
Herbicide: Terbacil is a selective herbicide that treats a broad spectrum of broadleaf weeds and grasses. It is formulated as a wettable powder and is applied by aircraft or ground equipment on terrestrial food and feed crops (e.g., apples, mint/peppermint/spearmint, sugarcane, and ornamentals), forestry [e.g., cottonwood (forest/shelterbelt)], terrestrialfood (e.g., asparagus, blackberry, boysenberry, dewberry, loganberry, peach, raspberry, youngberry and strawberry), and terrestrial feed (e.g., alfalfa, sainfoin (hay and fodder), and forage). Not approved for use in EU countries. Registered for use in the U.S.
Trade name
COMPOUND® 732; DuPontTM® 732; EXPERIMENTAL HERBICIDE 732; GEONTER®; SINBAR®; TURBSVIL®; ZOBAR®[C]
Environmental Fate
Soil. In microbially active soils, tebuthiuron is degraded via demethylation. The average
half-life in soil 12–15 months in areas receiving 60 inches of rainfall a year (Humburg et
al., 1989).
Groundwater. According to the U.S. EPA (1986) terbacil has a high potential to leach
to groundwater.
Plant. The major degradation products of [2-14C]terbacil identified in alfalfa using a
mass spectrometer were (% of applied amount): 3-tert-butyl-5-chloro-6-hydroxymethyluracil
(11.9) and 6-chloro-2,3-dihydro-7-(hydroxymethyl)-3,3-dimethyl-5H-oxa
Photolytic. Acher et al. (1981) studied the dye-sensitized photolysis of terbacil in
aerated aqueous solutions over a wide pH range. After a 2-hour exposure to sunlight,
terbacil in aqueous solution (pH range 3.0–9.2) in the presence of methylene blue (3 ppm)
or riboflavin (10 ppm), decomposed to 3-tert-5-butyl-5-acetyl-5-hydroxyhydantoin.
Deacylation was observed under alkaline conditions (pH 8.0 or 9.2) affording 3-tert-5-
hydroxyhydantoin. In neutral or acidic conditions (pH 6.8 or 3.0) containing riboflavin, a
mono-N-dealkylated terbacil dimer and an unidentified water-soluble product formed.
Product formation, the relative amounts of products formed and the rate of photolysis
were found to be all dependent upon pH, sensitizer, temperature and time (Acher et al.,
1981).
Chemical/Physical. Stable in water (Worthing and Hance, 1991).
Metabolic pathway
When rats are administered terbacil orally, the primary biotransformation products of terbacil in the urine are derived from hydroxylation of the 6-methyl group and are identified as the aglycone, glucuronide, and sulfate. The other conjugated metabolite is the mercapturic acid conjugate of the 6-methyl substituent. Minor metabolites are identified as sulfoxides and sulfone at the 6-methyl substituent of terbacil.
Properties of Terbacil
| Melting point: | 175-177° |
| Density | 1.3400 |
| refractive index | 1.5388 (estimate) |
| storage temp. | 0-6°C |
| form | Solid |
| pka | 8.79±0.40(Predicted) |
| color | Off-white |
| Water Solubility | 0.71g/L(25 ºC) |
| Merck | 13,9230 |
| BRN | 643054 |
| NIST Chemistry Reference | Terbacil(5902-51-2) |
| EPA Substance Registry System | Terbacil (5902-51-2) |
Safety information for Terbacil
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P501:Dispose of contents/container to..… |
Computed Descriptors for Terbacil
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1