Dibutyl phthalate
Synonym(s):DBP;Dibutyl phthalate;Red 2;Phthalic acid dibutyl ester;Dibenzo{[f,f′]-4,4′,7,7′-tetraphenyl}diindeno[1,2,3-cd:1′,2′,3′-lm]perylene
- CAS NO.:84-74-2
- Empirical Formula: C16H22O4
- Molecular Weight: 278.34
- MDL number: MFCD00009441
- EINECS: 201-557-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-20 21:15:22
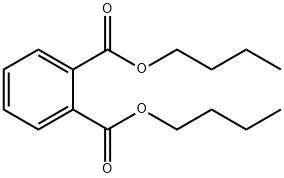
What is Dibutyl phthalate ?
Description
Dibutyl phthalate is an oily, sparingly water-soluble liquid with a very high boiling point (350℃). It is included as an insect repellent in some aerosol sprays used to treat flystrike in sheep.
Chemical properties
Dibutyl phthalate occurs as an odorless, oily, colorless, or very slightly yellow-colored, viscous liquid.
Physical properties
Colorless to pale yellow, oily, viscous liquid with a mild, aromatic odor
The Uses of Dibutyl phthalate
Dibutyl phthalate is used in plasticizers, cosmetics, safety glass, insecticides, printing inks, paper coatings, adhesives, elastomers and explosives; solvent in polysulfide dental impression materials; solvent for perfume oils; perfume fixative; textile lubricating agent; solid rocket propellent; emollient in aerosol antiperspirants; insect repeller; plasticizer in various plastic materials.
Production Methods
Dibutyl phthalate is produced from n-butanol and phthalic anhydride in an ester formation reaction.
Definition
ChEBI: A phthalate ester that is the diester obtained by the formal condensation of the carboxy groups of phthalic acid with two molecules of butan-1-ol.
General Description
Dibutyl phthalate is a colorless oily liquid, it is insoluble in water. It is used in paints and plastics and as a reaction media for chemical reactions.The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Since Dibutyl phthalate is a liquid Dibutyl phthalate can easily penetrate the soil and contaminate groundwater and nearby streams.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Dibutyl phthalate is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. Avoid contact with strong oxidizing agents and strong bases. Will not polymerize. [USCG, 1999]. Can generate electrostatic charges. [Handling Chemicals Safely 1980. p. 250].
Health Hazard
The toxicity of this compound is very low. Inhumans, oral intake of dibutyl phthalate at adose level of 150 mg/kg may cause nausea,vomiting, dizziness, hallucination, distortedvision, lacrimation, and conjunctivitis withprompt recovery. It metabolizes to monobutylester and phthalic acid and is excreted in urine.The inhalation toxicity should be insignificantbecause of its negligible low vapor pressure[<0.1 torr at 20°C (68°F)]. However, expo sure to its mist or aerosol can cause irritationof eyes and mucous membranes
LD50 value, oral (mice): 5300 mg/kg.
Fire Hazard
Combustible.
Flammability and Explosibility
Non flammable
Pharmaceutical Applications
Dibutyl phthalate is used in pharmaceutical formulations as a plasticizer in film-coatings. It has been evaluated as a pore-forming agent in novel delivery systems.It is also used extensively as a solvent, particularly in cosmetic formulations such as antiperspirants, hair shampoos, and hair sprays. In addition to a number of industrial applications, dibutyl phthalate is used as an insect repellent, although it is not as effective as dimethyl phthalate.
Safety Profile
Moderately toxic by intraperitoneal and intravenous routes. Mildly toxic by ingestion. Human systemic eye effects by ingestion, hallucinations, dstorted perceptions, nausea or vomiting, and kidney, ureter, or bladder changes. Experimental teratogenic and reproductive effects. Mutation data reported. Combustible when exposed to heat or flame; can react with oxidizing materials. Violent reaction with Cl2. Incompatible with chlorine. To fight fire, use CO2, dry chemical. When heated to decomposition it emits acrid smoke and fumes. See also ESTERS, PHTHALIC ACID, and n BUTYL ALCOHOL.
Safety
Dibutyl phthalate is generally regarded as a relatively nontoxic material, although it has occasionally been reported to cause hypersensitivity reactions. It is widely used in topical cosmetic and some oral pharmaceutical formulations.
LD50 (mouse, IV): 0.72g/kg
LD50 (mouse, oral): 5.3g/kg
LD50 (rat, oral): 8.0g/kg
LD50 (rat, IP): 3.05mL/kg
Potential Exposure
AgriculturalChemical; Tumorigen, Mutagen; Reproductive Effector;Human Data. Used in making vinyl compounds, inplasticizing vinyl acetate emulsion systems, and in plasticizing cellulose esters. Also used as a lacquer solvent (nail polish remover) and insect repellent.
First aid
If Dibutyl phthalate gets into the eyes, remove any contact lenses at once and irrigate immediately for at least 15 min,occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin, removecontaminated clothing and wash immediately with soap andwater. Seek medical attention immediately. If this chemicalhas been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask)if breathing has stopped and CPR if heart action has stopped.Transfer promptly to a medical facility. When this chemicalhas been swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit.
Source
Detected in distilled water-soluble fractions of new and used motor oil at concentrations of 38 to 43 and 15 to 23 μg/L, respectively (Chen et al., 1994). Leaching from flexible plastics in contact with water. Laboratory contaminant.
Environmental Fate
Biological. Under aerobic conditions using a freshwater hydrosol, mono-n-butyl phthalate and phthalic acid were produced. Under anaerobic conditions, phthalic acid was not present (Verschueren, 1983). In anaerobic sludge, dibutyl phthalate degraded as follows: monobutyl phthalate to phthalic acid to protocatechuic acid followed by ring cleavage and mineralization (Shelton et al., 1984).
Photolytic. An aqueous solution containing titanium dioxide and subjected to UV radiation (l >290 nm) produced hydroxyphthalates and dihydroxyphthalates as intermediates (Hustert and Moza, 1988).
Chemical/Physical. Pyrolysis of dibutyl phthalate in the presence of polyvinyl chloride at 600°C gave the following compounds: indene, methylindene, naphthalene, 1- methylnaphthalene, 2-methylnaphthalene, biphenyl, dimethylnaphthalene, acenaphthene, fluorene, methylacenaphthene, methylfluorene and six unidentified compounds (Bove and Dalven, 1984).
Under alkaline conditions, dibutyl phthalate will initially hydrolyze to n-butyl hydrogen phthalate and n-butanol. The monoester will undergo further hydrolysis forming o-phthalic acid and n-butanol (Kollig, 1993).
Storage
Dibutyl phthalate should be stored in a well-closed container in a cool, dry, location. Containers may be hazardous when empty since they can contain product residues such as vapors and liquids.
Shipping
Based on regulations, it may be classified asan Environmentally hazardous substances, liquid, n.o.s. Itfalls in Hazard Class 9 and Packing Group III.[20,21]
Purification Methods
Wash DBP with H2O (to free it from alcohol), then dilute NaOH (to remove any butyl hydrogen phthalate or acid), aqueous NaHCO3 (charcoal), then distilled water. Dry it (CaCl2), distil it at 10torr or less, and store it in a desiccator over P2O5. [Beilstein 9 II 586, 9 III 4102, 9 IV 3175.]
Toxicity evaluation
Acute oral LD50 for rats: >6,000 mg/kg
Incompatibilities
Dibutyl phthalate reacts violently with chlorine. It also reacts with oxidizing agents, acids, bases, and nitrates.
Regulatory Status
Included in the FDA Inactive Ingredients Database (oral capsules, delayed action, enteric coated, and controlled release tablets). Included in nonparenteral medicines licensed in the UK (oral capsules, tablets, granules; topical creams and solutions).
Properties of Dibutyl phthalate
| Melting point: | -35 °C (lit.) |
| Boiling point: | 340 °C (lit.) |
| Density | 1.043 g/mL at 25 °C (lit.) |
| vapor density | 9.6 (vs air) |
| vapor pressure | 1 mm Hg ( 147 °C) |
| refractive index | n |
| Flash point: | 340 °F |
| storage temp. | 2-8°C |
| solubility | Very soluble in alcohol, ether, acetone, benzene |
| form | Liquid |
| color | APHA: ≤10 |
| Specific Gravity | 1.049 (20/20℃) |
| Odor | odorless |
| Relative polarity | 0.272 |
| explosive limit | 0.47%, 236°F |
| Water Solubility | Slightly soluble. 0.0013 g/100 mL |
| FreezingPoint | -35℃ |
| Merck | 14,3035 |
| BRN | 1914064 |
| Henry's Law Constant | 6.3 x 10-5 atm?m3/mol (quoted, Petrasek et al., 1983) |
| Dielectric constant | 6.0(45℃) |
| Exposure limits | NIOSH REL: TWA 5 mg/m3, IDLH 4,000 mg/m3; OSHA PEL: TWA
5 mg/m3; ACGIH TLV: TWA 5 mg/m3. |
| CAS DataBase Reference | 84-74-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Dibutyl phthalate(84-74-2) |
| EPA Substance Registry System | Dibutyl phthalate (84-74-2) |
Safety information for Dibutyl phthalate
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P273:Avoid release to the environment. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Dibutyl phthalate
| InChIKey | DOIRQSBPFJWKBE-UHFFFAOYSA-N |
Dibutyl phthalate manufacturer
Mine Chem India
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Dibutyl phthalate supplier,High quality high purity, size packagingView Details
Dibutyl phthalate supplier,High quality high purity, size packagingView Details
84-74-2 -
 Dibutyl Phthalate (SQ) CAS 84-74-2View Details
Dibutyl Phthalate (SQ) CAS 84-74-2View Details
84-74-2 -
 Dibutyl Phthalate CASView Details
Dibutyl Phthalate CASView Details -
 Dibutyl Phthalate pure CAS 84-74-2View Details
Dibutyl Phthalate pure CAS 84-74-2View Details
84-74-2 -
 Dibutyl Phthalate CASView Details
Dibutyl Phthalate CASView Details -
 Dibutyl Phthalate Liquid, Packaging Type: Drum, Packaging Size: 100 LiterView Details
Dibutyl Phthalate Liquid, Packaging Type: Drum, Packaging Size: 100 LiterView Details
84-74-2 -
 Phthalates Dibutyl phthalate (DBP), For Industrial, Grade: Chemical GradeView Details
Phthalates Dibutyl phthalate (DBP), For Industrial, Grade: Chemical GradeView Details
84-74-2 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2
