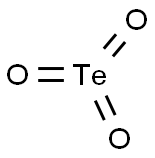
Tellurium dioxide
Synonym(s):Telluria;Tellurium dioxide;Tellurium oxide;Tellurium(IV) oxide
- CAS NO.:7446-07-3
- Empirical Formula: O2Te
- Molecular Weight: 159.6
- MDL number: MFCD00011263
- EINECS: 231-193-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
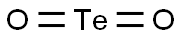
What is Tellurium dioxide?
Chemical properties
Heavy, white, crystalline powder; odorless. Soluble in concentrated
acids, alkalies; slightly soluble in dilute
acids, water.
Physical properties
White crystals; dimorphic; exists in tetragonal and orthorhombic forms; density 5.75 g/cm3 (tetragonal), 6.04 g/cm3 (orthorhombic); melts at 733°C forming a deep yellow liquid; vaporizes at 1,245°C; insoluble in water; soluble in acids and alkalies.
The Uses of Tellurium dioxide
Tellurium dioxide is also used in devices which can convert light into sound (acousto-optic material). Glasses made of tellurium oxide have high refractive indices and transmit into mid-IR region. It is used to make glasses with special properties. It is useful in optical waveguides and optical fiber amplification.
The Uses of Tellurium dioxide
Tellurium dioxide is used to prepare tellurium metal, telluric acid, and many tellurium salts. It may also be used in the preparation of Ag2Te nanoparticles.
The Uses of Tellurium dioxide
TeO2 can be potentially used in medical imaging and industrial monitoring processes.
Production Methods
Tellurium dioxide in its orthorhombic form occurs in nature as mineral tellurite. It is mined from natural deposits. Also, tellurium dioxide is produced as an intermediate during recovery of tellurium metal from anode slimes of electrolytic copper refining (See Tellurium, Production). The dioxide also is prepared by treating tellurium metal with hot nitric acid to form 2TeO2?HNO3. The product then is heated to drive off nitric acid.
General Description
Tellurium dioxide (TeO2) is a ceramic material that can be used as a semiconducting oxide. It has a wide band gap and high mobility as determined by density functional theory (DFT) calculations. In bulk quantity, it exists in two polymorphs which include tetragonal α-TeO2 and orthorhombic β-TeO2.
Flammability and Explosibility
Non flammable
Safety Profile
Poison by intratracheal route. An experimental teratogen. Experimental reproductive effects. When heated to decomposition it emits toxic fumes of Te. See also TELLURIUM COMPOUNDS.
Purification Methods
Dissolve it in 5M NaOH, filter it and precipitate it by adding 10M HNO3 to the filtrate until the solution is acid to phenolphthalein. After decanting the supernatant, the precipitate is washed five times with distilled water, then dried for 24hours at 110o [Horner & Leonhard J Am Chem Soc 74 3694 1952].
Properties of Tellurium dioxide
| Melting point: | 733 °C (lit.) |
| Boiling point: | 1245 °C |
| Density | 5.67 g/mL at 25 °C (lit.) |
| vapor pressure | 0Pa at 25℃ |
| storage temp. | no restrictions. |
| solubility | insoluble in H2O; soluble in alkaline solutions, acid solutions |
| form | Powder |
| color | White to slightly yellow |
| Specific Gravity | 5.8 |
| Water Solubility | insoluble |
| λmax | 360nm(neat)(lit.) |
| Merck | 14,9123 |
| Exposure limits | ACGIH: TWA 0.1 mg/m3 NIOSH: IDLH 25 mg/m3; TWA 0.1 mg/m3 |
| Stability: | Stable. Incompatible with strong acids, strong oxidizing agents. |
| CAS DataBase Reference | 7446-07-3(CAS DataBase Reference) |
| NIST Chemistry Reference | tellurium(IV) dioxide(7446-07-3) |
| EPA Substance Registry System | Tellurium oxide (TeO2) (7446-07-3) |
Safety information for Tellurium dioxide
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H332:Acute toxicity,inhalation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Tellurium dioxide
Tellurium dioxide manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Tellurium(IV) Oxide CAS 7446-07-3View Details
Tellurium(IV) Oxide CAS 7446-07-3View Details
7446-07-3 -
 Tellurium(IV) oxide CAS 7446-07-3View Details
Tellurium(IV) oxide CAS 7446-07-3View Details
7446-07-3 -
 Tellurium(IV) oxide CAS 7446-07-3View Details
Tellurium(IV) oxide CAS 7446-07-3View Details
7446-07-3 -
 Tellurium(IV) oxide, (metals basis) CAS 7446-07-3View Details
Tellurium(IV) oxide, (metals basis) CAS 7446-07-3View Details
7446-07-3 -
 Tellurium(IV) Oxide CAS 7446-07-3View Details
Tellurium(IV) Oxide CAS 7446-07-3View Details
7446-07-3 -
 Tellurium(IV) Oxide CAS 7446-07-3View Details
Tellurium(IV) Oxide CAS 7446-07-3View Details
7446-07-3 -
 Tellurium Dioxide ultrapure CAS 7446-07-3View Details
Tellurium Dioxide ultrapure CAS 7446-07-3View Details
7446-07-3 -
 Tellurium oxide, ≥97% CAS 7446-07-3View Details
Tellurium oxide, ≥97% CAS 7446-07-3View Details
7446-07-3