sulfur dioxide
- CAS NO.:89125-89-3
- Empirical Formula: O2S
- Molecular Weight: 64.06
- MDL number: MFCD00011450
- SAFETY DATA SHEET (SDS)
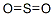
What is sulfur dioxide?
Chemical properties
Sulfur dioxide is a noncombustible colorless gas at ambient temperatures with a characteristic, strong, suffocating odor. The Odor Threshold is 1.1ppm. Shipped as a liquefied compressed gas.
Potential Exposure
Sulfur dioxide is used in the manufacture of sodium sulfite, sulfuric acid; sulfuryl chloride; thionyl chloride; organic sulfonates; disinfectants, fumigants, glass, wine, ice, industrial and edible protein; and vapor pressure thermometers. It is also used in the bleaching of beet sugar, flour, fruit, gelatin, glue, grain, oil, straw, textiles, wicker ware; wood pulp; and wool; in the tanning of leather; in brewing and preserving; and in the refrigeration industry. Exposure may also occur in various other industrial processes as it is a by-product of ore smelting, coal and fuel oil combustion; paper manufacturing and petroleum refining.
Shipping
UN1079 Sulfur dioxide, Hazard Class: 2.3; Labels: 2.3-Poisonous gas, 8-Corrosive material, Inhalation Hazard Zone C. Cylinders must be transported in a secure upright position, in a well-ventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written permission of the owner
Incompatibilities
Reacts with water to form sulfurous acid, a medium-strong acid. Reacts violently with ammonia, acrolein, acetylene; alkali metals; such as sodium, potassium, magnesium, and zinc; chlorine, ethylene oxide; amines, butadiene. Attacks many metals including aluminum, iron, steel, brass, copper, nickel; especially in presence of water or steam. Incompatible with halogens. Attacks plastics, rubber and coatings.
Waste Disposal
Return refillable compressed gas cylinders to supplier. Pass into soda ash solution, then add calcium hypochlorite; neutralize and flush to sewer with water (A-38).
Safety information for sulfur dioxide
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8