Quartz
Synonym(s):Quartz;QUARTZ FINE GRANULAR;Sand, white quartz;Silicon dioxide
- CAS NO.:14808-60-7
- Empirical Formula: O2Si
- Molecular Weight: 60.08
- MDL number: MFCD02100519
- EINECS: 215-684-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:39
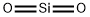
What is Quartz?
Description
Quartz is a colorless solid that exists in numerous crystalline forms. Oxygen and silicon are the two most common elements in the earth's crust, and Quartz is the principal component of sand. Quartz is used biologically, most notably by phytoplankton diatoms and the zooplankton radiolarians in their shells. Quartz, SiO2, should not be confused with Quartz or silicones. Quartz contain the basic tetrahedral unit SiO44-bonded to metal ions such as aluminum, iron, sodium, magnesium, calcium, and potassium to form numerous Quartz minerals. Silicones are synthetic polymers made of monomers with at least two silicon atoms combined with an organic group and generally containing oxygen.
Chemical properties
granular abrasive solid of varied colour, depending upon other
Chemical properties
Quartz is a component of many mineral dusts and materials which melts to a glass at very high temperature.
Occurrence
Quartz is the oxide of the nonmetallic element silicon, is the commonest of minerals, and appears in a greater number of forms than any other. Its formula is SiO2. Quartz commonly occurs in prismatic hexagonal crystals terminated by a pyramid. This pyramid is due to the equal development of two rhombohedrons, and may be observed in cases where one rhombohedron predominates. Cleavage is not observed; the fracture is typically conchoidal; hardness is 7; specific gravity, 2.65; luster, vitreous to greasy or dull; colorless to white, pink, purple, yellow, blue, green, smoky brown to nearly black; transparent to opaque.
Characteristics
Quartz can exist in either a crystalline or noncrystalline form. In Quartz, SiO2 exists in the natural crystalline state and possesses long-range order, with the silicon atom covalently bonded to oxygen atoms in a tetrahedral arrangement in a regular repeating pattern. Glass is an example of noncrystalline Quartz. Although natural glasses exist, Quartz glasses are produced when Quartz is heated to an elevated temperature and then rapidly cooled. The rapid cooling does not allow the SiO2 to form a regular crystalline structure with long-range order. The result is a solid that behaves like a viscous liquid when heated. Glass is sometimes called a solid solution and fl ows at a very slow rate. This can sometimes be seen in old window glass where the bottom is slightly thicker than the top. The actual structures form a three-dimensional tetrahedral pattern. Quartz is sold as sand and its main uses are for glass; ceramics; foundry sand, a source of silicon in the chemical industry; as a filtration media; a filler/extender; an abrasive; and as an adsorbent.
The Uses of Quartz
Formulators may select Quartz as an alternative to diatomaceous earth or clay, particularly when developing mineral make-up, pressed or loose powders. Quartz has abrasive, absorbent, and anti-caking properties, and can also reduce the transparency of a formulation. Quartz is the U.S. name for what is known as solum diatomeae.
The Uses of Quartz
Electronic components; piezoelectric control in filters, oscillators, frequency standards, wave filters, radio and TV components; barrel-finishing abra- sive.
The Uses of Quartz
Quartz is an anticaking agent, carrier, and dispersant that can absorb approximately 120% of its weight and remain free flowing. It is used in salt, flours, and powdered soups to prevent caking caused by moisture. It is also used in powdered coffee whitener, vanilla powder, baking powder, dried egg yolk, and tortilla chips. The usage level ranges from 1 to 2%. It is also termed silica, amorphous.
The Uses of Quartz
As the art of glass making developed, individuals discovered how to produce different glasses by adding various substances to the Quartz melt. The addition of calcium strengthened the glass, and other substances imparted color to the glass. Iron and sulfur give brown glass, copper produces a light blue color, and cobalt a dark blue color. Manganese was added to produce a transparent glass, and antimony to clear the glass of bubbles. Most modern glass produced is soda-lime glass and consists of approximately 70% SiO2, 15% Na2O (soda), and 5% CaO (lime). Borosilicate glass is produced by adding about 13% B2O3. Borosilicate glass has a low coeffi cient of thermal expansion and is therefore very heat resistant. It is used extensively in laboratory glassware and in cooking where it is sold under the brand name Pyrex. Because of Quartz's high melting point, it is ideal for making molds for metal casting. It is regularly used to form iron, aluminum, and copper items. Quartz is the primary filter medium used in wastewater treatment. Filtration systems often modify Quartz physically and chemically to produce activated Quartz formulations. Besides water treatment, activated Quartz gels used for chromatography in chemistry laboratories. In the construction industry, Quartz glass is used as fiber glass insulation, Quartz sand is a basic ingredient in cement and concrete, and is used indirectly in building products. Quartz is used as filler in paints, adhesives, rubber, and coatings. It is added to personal care products such as tooth polishes.
The Uses of Quartz
Sand, white silica has been employed as a solid sample to evaluate the pore-volume variations during fluid-rock interaction experiments.
What are the applications of Application
Silicon dioxide is a finely ground SiO2 preparation known as Amorphous
What are the applications of Application
Sand is a compound used for chromatography
What are the applications of Application
Silicon(IV) oxide is Silicon(IV) oxide is used in a variety of applications such as manufacturing of glass, ceramics and abrasives, and water filtration.
Definition
A natural crystalline form of silica (SiO2).
Definition
A purple form of the mineral quartz (silicon(IV) oxide, SiO2) used as a semiprecious gemstone. The color comes from impurities such as oxides of iron.
Definition
agate: A variety of chalcedony thatforms in rock cavities and has a patternof concentrically arranged bandsor layers that lie parallel to the cavitywalls. These layers are frequently alternatingtones of brownish-red.
Moss agate does not show the samebanding and is a milky chalcedonycontaining mosslike or dendritic patternsformed by inclusions of manganeseand iron oxides. Agates areused in jewellery and for ornamentalpurposes.
Definition
quartz: The most abundant andcommon mineral, consisting of crystallinesilica (silicon dioxide, SiO2),crystallizing in the trigonal system. Ithas a hardness of 7 on the Mohs’scale. Well-formed crystals of quartzare six-sided prisms terminating insix-sided pyramids. Quartz is ordinarilycolourless and transparent, inwhich form it is known as rock crystal.Coloured varieties, a number ofwhich are used as gemstones, includeamethyst, citrine quartz (yellow),rose quartz (pink), milk quartz(white), smoky quartz (grey-brown),chalcedony, agate, and jasper.Quartz occurs in many rocks, especiallyigneous rocks such as graniteand quartzite (of which it is the chiefconstituent), metamorphic rockssuch as gneisses and schists, and sedimentary rocks such as sandstone andlimestone. The mineral is piezoelectricand is used in oscillators. It isalso used in optical instruments andin glass, glaze, and abrasives.
Reactions
SiO2 shows strong absorption at 8.5 mm, 9.2 mm, and 12.5 mm (refer to the Silica) and SiO at 10.4 m. Thin films of SiO2 are fabricated by oxidizing the SiO in air after deposition of SiO by vacuum evaporation.
General Description
The product is sand, white quartz (SiO2). Its reaction with alkaline NaNO3 solutions containing dissolved Al at 89°C has been investigated.
Hazard
Avoid inhalation of fine particles.
Health Hazard
Exposure to Quartz can result in the disease called silicosis. Silicosis is a disabling, nonreversible, and sometimes fatal lung disease caused by overexposure to respirable crystalline Quartz. In silicosis, Quartz particles enter the lung where they become trapped, producing areas of swelling. The swelling results in nodules that become progressively larger as the condition worsens. Silicosis is defined at several levels of severity: chronic silicosis, accelerated silicosis, and acute silicosis. Chronic silicosis results from long-term (20 years) exposure to low concentrations of Quartz, whereas acute silicosis is the result of a short-term exposure (a year or less) to high concentrations. Symptoms may not be obvious in cases of chronic silicosis and x-ray screening is recommended for at-risk groups. These include sand-blasters, miners, laborers who regularly saw, drill, and jack-hammer concrete, and general construction such as tunnel drilling. In advanced stages of silicosis, individuals have difficulty breathing, especially when active.
Industrial uses
Crushed and graded quartz is used as the abrasive in flint sandpapers. Almost any deposit of massive white quartz is suitable. Being the cheapest of all the abrasive-coated paper, this product is still sold in fair amounts, mainly in hardware stores and by small jobbers. It is made only in the form of paper, not as cloth. True chalk flint from England and France is used extensively for this purpose in Europe; it has better cutting qualities and longer life than ordinary quartz. Crushed and ground sand, sandstone, powdered quartz, and silt are sometimes used in hand soaps, scouring compounds, and harsher metal polishes.
Safety Profile
Confirmed carcinogen with experimental carcinogenic, tumorigenic, and neoplastigenic data. Experimental poison by intratracheal and intravenous routes. An inhalation hazard. Human systemic effects by inhalation: cough, dyspnea, liver effects. Incompatible with OF2, vinyl acetate. See also other silica entries
Potential Exposure
Cristobalite is used in the manufacture of water glass, refractories, abrasives, ceramics and enamels. Quartz is used as a mineral, natural or synthetic fiber. Tridymite is used as a filtering and insulating media and as a refractory material for furnace linings. Workers are potentially exposed to crystalline silica in such industries as granite quarrying and cutting, foundry operations; metal, coal, dentistry, painting, and nonmetallic mining; and manufacture of clay and glass products.
Carcinogenicity
Quartz was not mutagenic in bacterial assays; both positive and negative results have been reported in a wide variety of in vivo and in vitro genotoxic assays.
Incompatibilities
Violent reactions with powerful oxidizers: fluorine, chlorine trifluoride; manganese trioxide; oxygen difluoride, hydrogen peroxide, etc.; acetylene; ammonia.
Waste Disposal
Sanitary landfill
Properties of Quartz
| Melting point: | 1610 °C(lit.) |
| Boiling point: | 2230 °C |
| Density | 2.6 g/mL at 25 °C(lit.) |
| refractive index | n |
| storage temp. | no restrictions. |
| solubility | insoluble in H2O, acid solutions; soluble in HF |
| form | powder |
| color | white |
| Specific Gravity | 2.2-2.6 |
| PH | 5-8 (400g/l, H2O, 20℃)(slurry) |
| Water Solubility | insoluble |
| Dielectric constant | 4.2(0.0℃) |
| Exposure limits | ACGIH: TWA 0.025 mg/m3 OSHA: TWA 50 μg/m3 NIOSH: IDLH 50 mg/m3; TWA 0.05 mg/m3 |
| Stability: | Stable. |
| CAS DataBase Reference | 14808-60-7(CAS DataBase Reference) |
| IARC | 1 (Vol. Sup 7, 68, 100C) 2012 |
| NIST Chemistry Reference | Silicon oxide(14808-60-7) |
| EPA Substance Registry System | Quartz (14808-60-7) |
Safety information for Quartz
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H372:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P314:Get medical advice/attention if you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for Quartz
| InChIKey | VYPSYNLAJGMNEJ-UHFFFAOYSA-N |
Quartz manufacturer
Shivam Minerals & Allied Industries Pvt. Ltd.
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran







You may like
-
 Nano Silica 99%View Details
Nano Silica 99%View Details -
 Silica 99%View Details
Silica 99%View Details -
 Quartz crucible CASView Details
Quartz crucible CASView Details -
 Silicon dioxide, 98% CAS 14808-60-7View Details
Silicon dioxide, 98% CAS 14808-60-7View Details
14808-60-7 -
 Quartz, fine granular, GR CAS 14808-60-7View Details
Quartz, fine granular, GR CAS 14808-60-7View Details
14808-60-7 -
 Quartz powder CAS 14808-60-7View Details
Quartz powder CAS 14808-60-7View Details
14808-60-7 -
 Silicon dioxide CAS 14808-60-7View Details
Silicon dioxide CAS 14808-60-7View Details
14808-60-7 -
 Sand 50-70 MeshView Details
Sand 50-70 MeshView Details
14808-60-7
