Tapinarof
- CAS NO.:79338-84-4
- Empirical Formula: C17H18O2
- Molecular Weight: 254.32
- MDL number: MFCD13193203
- EINECS: 1312995-182-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-05-27 21:43:18
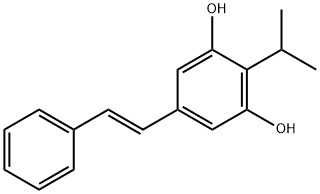
What is Tapinarof?
Absorption
No accumulation was observed with repeat topical application. Plasma concentration of tapinarof was below the quantifiable limits (BQL) of the assay (lower limit of quantification was 50 pg/mL) in 68% of the pharmacokinetic samples. On Day 1, mean ± SD values of Cmax and AUC0-last were 0.90 ± 1.4 ng/mL and 4.1 ± 6.3 ng.h/mL, respectively, following a mean daily dose of 5.23 g applied to a mean body surface area involvement of 27.2% (range 21 to 46%) in 21 subjects with moderate to severe plaque psoriasis. On Day 29, the mean ± SD Cmax and AUC0-last were 0.12 ± 0.15 ng/mL and 0.61 ± 0.65 ng.h/mL, respectively.
Toxicity
Long-term carcinogenicity studies were conducted in mice (daily topical administration at doses of 0.5, 1.5, and 3% tapinarof cream) and in rats (subcutaneous administration at doses of 0.1, 0.3, and 1 mg/kg/day tapinarof). No drug-related neoplasms were noted in mice after 98 (females) to 102 (males) weeks of daily topical administration at doses up to 3% tapinarof cream (44 times the MRHD based on AUC comparisons). No drug-related neoplasms were noted in female rats after 83 weeks of daily subcutaneous administration at up to 1 mg/kg/day tapinarof (9 times the MRHD based on AUC comparisons).
Tapinarof revealed no evidence of mutagenicity or clastogenicity in an Ames assay, an in vitro mammalian chromosomal aberration assay, an in vitro mouse lymphoma assay, and two in vivo micronucleus assays in mice and rats.
Tapinarof did not impair female fertility at subcutaneous doses up to 30 mg/kg/day (268 times the MRHD based on AUC comparisons).
Indications
Tapinarof is indicated for the topical treatment of plaque psoriasis in adults.
Background
Tapinarof is a novel, first-in-class, small-molecule AhR agonist that is indicated for the treatment of adult psoriasis. It is available as a topical cream to be applied to the affected area once daily. Tapiranof was first discovered as a metabolite (3,5-dihydroxy-4-isopropylstilbene) produced in Photorhabdus luminescens, a gram-negative bacillus that lives symbiotically with the Heterorhabditis nematodes. In 1959, it was noticed that Heterorhabditis with a high amount of 3,5-dihydroxy-4-isopropylstilbene did not putrefy once dead, thus suggesting its potential anti-inflammatory activity.
Tapinarof received initial approval from the FDA in 2022.
Pharmacokinetics
The pharmacodynamics of tapinarof are unknown.
Clinical Use
Tapinarof is a novel small molecule topical therapeutic AhR ( aryl hydrocarbon receptor)-modulating agent that has been recently approved by the FDA for the topical treatment of plaque psoriasis in adults. Tapinarof 1% cream has shown to be effective and to have a favorable safety profile in the treatment of psoriatic patients[1]. It could decrease the expression of multiple essential cytokines involved in the pathological IL-23/IL-17/IL-22 axis and ameliorate IMQ-induced psoriatic dermatitis, inhibiting keratinocyte proliferation and abnormal differentiation. But tapinarof may have different effects on varied types of psoriasis[2]. Tapinarof can also specifically bind to and activate AHR leading to downregulation of TNF-α/IL-23/IL-17 and inhibition of IL-4/IL-13 mediated STAT6 activation, to achieve the purpose of treating nonulcerated necrobiosis lipoidica[3].
in vivo
Tapinarof acts through AhR to reduce inflammation in IMQ-treated mice. AhR-sufficient mice on a C57Bl/6 background exhibit a reduced clinical score after treatment with Tapinarof or 6-formylindolo(3,2-b)carbazole (FICZ). In contrast, AhR KO mice do not respond to the anti-inflammatory effects of Tapinarof. FICZ is used as a comparator in these studies and yields similar results, with dramatically reduced inflammatory responses in wild-type, but not AhR KO mice.
Metabolism
Tapinarof is metabolized in the liver by multiple pathways including oxidation, glucuronidation, and sulfation in vitro.CYP1A2 and CYP3A4 appears to be the major enzyme involved in the hepatic metabolism of tapinarof, while CYP2C9, CYP2C19, and CYP2D6 play a minor role.
References
[1] Bissonnette R, et al. Tapinarof for psoriasis and atopic dermatitis: 15?years of clinical research. Journal of the European Academy of Dermatology and Venereology, 2023; 37: 1168-1174.
[2] Zhu X, et al. The opposite effect of tapinarof between IMQ and IL-23 induced psoriasis mouse models. Experimental Dermatology, 2023.
[3] Palomares S, et al. Nonulcerated Necrobiosis Lipoidica Successfully Treated with Tapinarof: A Case Report. Clinical, Cosmetic and Investigational Dermatology, 2023; 16: 1373-1376.
Properties of Tapinarof
| Melting point: | 140 - 142°C |
| Boiling point: | 431.8±20.0 °C(Predicted) |
| Density | 1.158 |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 9.86±0.15(Predicted) |
| color | Pale Brown to Light Brown |
Safety information for Tapinarof
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Tapinarof
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 Benvitimod CAS 79338-84-4View Details
Benvitimod CAS 79338-84-4View Details
79338-84-4 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
