Polystyrene
Synonym(s):Polystyrene;PS;PS-COOH;
- CAS NO.:9003-53-6
- Empirical Formula: [CH2CH(C6H5)]n
- Molecular Weight: 2.01588
- MDL number: MFCD00084450
- EINECS: 202-851-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:44
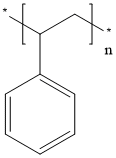
What is Polystyrene?
Chemical properties
Poly(styrene) is white powder or beads, or clear solid.Polystyrene is rigid with excellent dimensional stability, has good chemical resistance to aqueous solutions, and is an extremely clear material.
Impact polystyrene contains polybutadiene added to reduce brittleness. The polybutadiene is usually dispersed as a discrete phase in a continuous polystyrene matrix. Polystyrene can be grafted onto rubber particles, which assures good adhesion between the phases.
Chemical properties
The specific gravity of general purpose and impact polystyrene is 1.05. It can vary for copolymers. It is higher for some specialty grades. Density varies slightly with pressure, but for practical purposes, the polymer is noncompressible. In terms of heat-resistance, deflection temperatures range from about 66 to 99 °C (170 to 215 °F), depending upon the formulation. Continuous resistance to heat for polystyrene is usually 60 to 80 °C (140 to 175 °F). Time and load have a significant influence on the useful service temperature of a part.
Polystyrene is nontoxic when free from additives and residuals. It has no nutritive value and does not support fungus or bacterial growth. Dimensional stability of polystyrene resins is excellent. Mold shrinkage is small. The low moisture absorption (about 0.02%) allows fabricated parts to maintain dimensions and strength in humid environments.
General-purpose polystyrene is water white, and transmission of visible light is about 90%. Modifiers reduce this property, and translucence results. The refractive index is about 1.59; critical angle about 39. Polystyrene molecules do not have the same optical properties in all directions. When molecules become oriented in a given direction during fabrication, a double refraction occurs and a birefringence effect can be observed if the part is examined through a polarized lens under a polarized light source. Injection moldings often exhibit birefringence in a random pattern.
The Uses of Polystyrene
Containers, tubs, and trays formed from extruded impact polystyrene sheets are used for packaging a large variety of food. Biaxially oriented polystyrene film is thermoformed into blister packs, meat trays, container lids, and cookie, candy, pastry, and other food packages where clarity is required.
Housewares is another large segment of the use of polystyrenes. Refrigerator door liners and furniture panels are typical thermoformed impact polystyrene applications. Extruded profiles of solid or foamed impact polystyrene are used for mirror or picture frames, and moldings for construction applications.
General-purpose polystyrene is extruded either clear or embossed for room dividers, shower doors, glazings, and lighting applications. Injection molding of impact polystyrene is used for household items, such as flower pots, personal care products, and toys. General-purpose polystyrene is used for cutlery, bottles, combs, disposable tumblers, dishes, and trays. Injection blow molding can be used to convert polystyrene into bottles, jars, and other types of open containers.
Impact polystyrene with ignition-resistant additives is used for appliance housings, such as those for television and small appliances. Structural foam impact polystyrene modified with flame-retardant additives is used for business machine housings and in furniture because of its decorability and ease of processing. Consumer electronics, such as cassettes, reels, and housings, is a fast growing area for use of polystyrenes. Medical applications include sample collectors, petri dishes, and test tubes. In an effort to make homes and other buildings more energy efficient, the use of polystyrenes in extruded foam board with flame-retardant additives for walls and under slabs has experienced exceptional growth in recent years. Used as a sheeting material, extruded foam board complies with the requirements of the major building codes as well as federal and military specifications.
The Uses of Polystyrene
Packaging film; molded parts for automobiles, appliances, housewares, etc.; wire and cable coat- ing; food container closures; coated and laminated products; bottles; artificial grass and turfs; plastic pipe; wearing apparel (acid-dyed); fish nets; sur- gical casts; strapping; synthetic paper; reinforced plastics; nonwoven disposable filters.
The Uses of Polystyrene
Polystyrene latex microspheres are utilized in flow cytometry, fluorescence microscopy, and as calibration particles. It finds a use in the detection of trace amounts of antigens or antibodies in serum, urine, and cerebro-spinal fluids. It is also employed as a stimulus responsive particulate emulsifier for oil-in-water emulsion.
Definition
ChEBI: A polymer composed of repeating ethyl benzene groups.
Definition
A synthetic polymer made from styrene (phenylethene). Expanded polystyrene is a rigid foam used in packing and insulation.
Definition
poly styrene: A clear glasslike materialmanufactured by free-radicalpolymerization of phenylethene(styrene) using benzoyl peroxide asan initiator. It is used as both a thermaland electrical insulator and forpacking and decorative purposes.
Preparation
Styrene may be polymerized by means of all four techniques by bulk, solution, suspension and emulsion polymerization.
Each of these methods is practised commercially, but solution polymerization is now the most extensively used. The four processes are described below.
(a) Bulk polymerization
In a common type of process, styrene is partially polymerized batch-wise by
heating the monomer (without added initiator) in large vessels at about 80°C
for 2 days until about 35% conversion is attained. The viscous solution of
polymer in monomer is then fed continuously into the top of a tower which is
some 25 feet high. The top of the tower is maintained at a temperature of
about 100°C, the centre at about 150°C and the bottom at about 180°C. As
the feed material traverses the temperature gradient, polymerization occurs
and fully polymerized material emerges from the base of the tower. The
reaction is controlled by a complex array of heating and cooling jackets and
coils with which the tower is fitted. The molten material is fed into an
extruder, extruded as filament and then cooled and chopped into granules.
Since the product contains few impurities, it has high clarity and good
electrical insulation properties. The polymer has a broader molecular weight
distribution than polymer prepared at one temperature.
(b) Solution polymerization
Continuous solution processes have found wide commercial utilization, the
main advantage over bulk methods being a lessening of the problems
associated with the movement and heat transfer of viscous masses. However,
the technique does require the added steps of solvent removal and recovery.
Typically, a mixture of monomer, solvent (3-12% ethylbenzene) and initiator
is fed into a train of three polymerization reactors, each with several heating
zones. The reaction temperature is progressively increased, rising from
110-130°C in the first reactor to 150-170°C in the last. The polymer solution
is then extruded as fine strands into a devolatilizing vessel. In this vessel,
which is at a temperature of 225°C, removal of solvent and unreacted
monomer takes place, being aided by the large surface area of the strands.
The molten material is fed into an extruder, extruded as filament, cooled and
chopped. It may be noted that this type of process is commonly regarded as a
continuous bulk process since the amount of solvent used is so small.
(c) Suspension polymerization
Suspension processes simplify the heat transfer problems associated with
bulk methods and, unlike solution methods, they do not involve solvent
removal and recovery. The disadvantages of the suspension technique are
that it requires the added step of drying and it does not readily lend itself to
continuous operation. Typically, polymerization is carried out batch-wise in
a stirred reactor, jacketed for heating and cooling.
Reaction temperature is about 90°C. When polymerization is complete, the
product, in the form of a slurry, is washed with hydrochloric acid and water
to remove suspending agent, centrifuged, dried in warm air (at about 60°C),
extruded and chopped.
(d) Emulsion polymerization
Emulsion processes are not used for making solid grades of polystyrene. This
is because these processes lead to polymer containing large quantities of soap
residues which impair the electrical insulation properties and optical clarity.
Emulsion polymerization does, however, find limited application in the
production of polystyrene latex used in water-based surface coatings. The
techniques employed are very similar to those used for other polymer latices,
e.g. poly(vinyl acetate) latex.
General Description
Polystyrene (for GPC, 4,000) is a synthetic thermoplastic, that is attractive for a wide range of applications because of its properties such as low cost, rigidity, low specific weight, high chemical resistance, mechanical flexibility, biocompatibility and good processability.
Hazard
Questionable carcinogen.
Industrial uses
Polystyrene is brittle at room temperature,becomes soft at 80°C, and is often modified bycopolymerization. Traditionally, it is used infilm form for capacitors, and it remains competitivefor this application. Poly styrene is alsoused for coaxial-cable insulation, but in woundstrip or bead form, because the solid is not veryflexible.
Safety Profile
Questionable carcinogen with experimental tumorigenic data by implant. When heated to decomposition it emits acrid smoke and irritating fumes. See also POLYMERS, IN SOLUBLE.
Purification Methods
Precipitate polystyrene repeatedly from CHCl3 or toluene solution by addition of MeOH. Dry it in vacuo. [Miyasaka et al. J Phys Chem 92 249 1988.]
Properties of Polystyrene
| Melting point: | 212 °C |
| Boiling point: | 30-80 °C |
| Density | 1.06 g/mL at 25 °C |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly, Sonicated) |
| form | powder |
| color | White |
| Water Solubility | insoluble |
| Dielectric constant | 24.0(Ambient) |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 9003-53-6 |
| IARC | 3 (Vol. 19, Sup 7) 1987 |
| NIST Chemistry Reference | Polystyrene(9003-53-6) |
| EPA Substance Registry System | Polystyrene (9003-53-6) |
Safety information for Polystyrene
Computed Descriptors for Polystyrene
| InChIKey | ZJMWRROPUADPEA-UHFFFAOYSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Polystyrene CASView Details
Polystyrene CASView Details -
 Polystyrene CASView Details
Polystyrene CASView Details -
 Polystyrene CASView Details
Polystyrene CASView Details -
 Polystyrene CASView Details
Polystyrene CASView Details -
 Polystyrene CASView Details
Polystyrene CASView Details -
 Polystyrene CASView Details
Polystyrene CASView Details -
 Polystyrene CASView Details
Polystyrene CASView Details -
 Polystyrene CASView Details
Polystyrene CASView Details
