Iguratimod
Synonym(s):3-Formylamino-7-methylsulfonylamino-6-phenoxy-4H-1-benzopyran-4-one;IGU;N-(7-(Methylsulfonamido)-4-oxo-6-phenoxy-4H-chromen-3-yl)formamide;N-[3-(Formylamino)-4-oxo-6-phenoxy-4H-1-benzopyran-7-yl]methanesulfonamide;T-614
- CAS NO.:123663-49-0
- Empirical Formula: C17H14N2O6S
- Molecular Weight: 374.37
- MDL number: MFCD00882374
- EINECS: 808-127-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-20 15:18:15
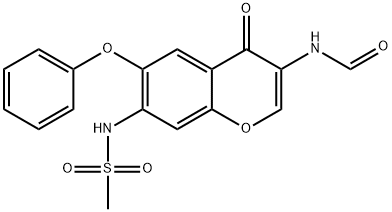
What is Iguratimod?
Description
In August 2011, China’s State FDA approved Simcere Pharmaceutical Group’s new drug application for iguratimod (T-614), a disease modifying anti-rheumatic drug (DMARD) for the treatment of rheumatoid arthritis (RA). Preclinical in vivo studies indicated that iguratimod was effective in an established adjuvant-induced arthritis model (ED40=3.6 mg/kg) in rats and also efficacious in a type II collagen-induced arthritis model in DBA/1J mice at 30 mg and 100 mg/kg.
Originator
Toyama (Japan)
The Uses of Iguratimod
Iguratimod acts as an anti-inflammatory agent, used primarily in the treatment of rheumatoid arthritis.
Definition
ChEBI: Iguratimod is an organic molecular entity.
brand name
Iremod
Clinical Use
Iguratimod, which was discovered by Toyama Pharmaceuticals and jointly co-developed with Eisai in Japan, was approved by the PMDA (Pharmaceuticals and Medical Devices Agency) of Japan on June 29, 2012 for the treatment of rheumatoid arthritis. This drug was also independently developed by Simcere Pharmaceutical Group and is marked as Iremod® in China. The drug exhibited inhibitory effects on granuloma inflammation, and was shown to be efficacious for the prevention of joint destruction in adjuvant arthritis.
Synthesis
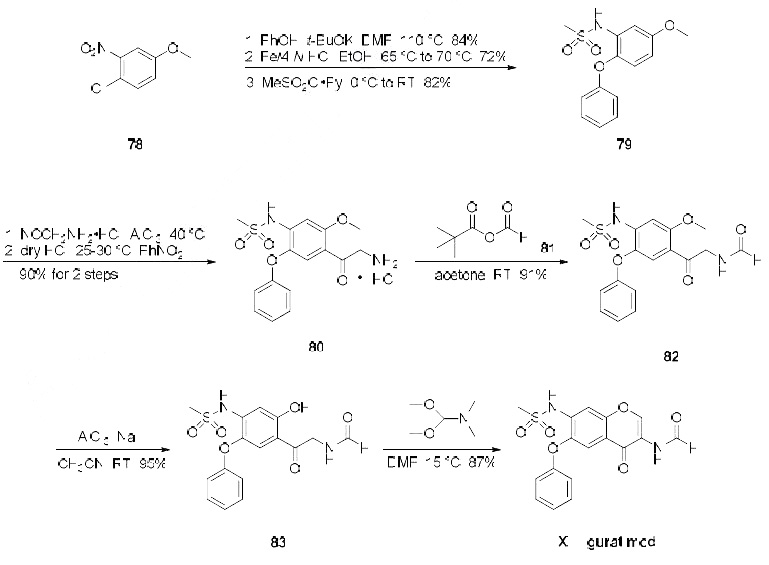
Several synthesis of iguratimod have been published, the most likely scale synthesis, which does not require chromatographic purification, is described in the scheme.The synthesis began with commercially available 3-nitro-4-chloro anisole (78) which was reacted with potassium phenoxide (generated from phenol and potassium t-butoxide at 110 oC) to provide the corresponding nitrophenyl ether which was subsequently reduced and sulfonylated to furnish sulfonamide 79. Next, this diphenyl ether was subjected to a Friedel-Crafts reaction with aminoacetonitrile hydrochloride which gave rise to aminomethylacetophenone 80 in 90% yield. This aminoketone was then formylated with formic trimethylacetic anhydride 81 at room temperature to afford formamide 82 in 91% yield, and this material was immediately subjected to O-demethylation conditions with aluminum trichloride and sodium iodide in acetonitrile to give the phenol 83 in 95% yield. Finally, treatment of the aminomethyl acetophenone phenol 83 with N,N-dimethylformamide dimethylacetal in DMF at low temperatures furnished iguratimod (XII) in 87% yield.
in vitro
iguratimod inhibited the release of immunoreactive il-1 beta from human monocytic cell line stimulated with lipopolysaccharides (lps) in a dose-dependent manner (0.3-30 μg/ml). northern blotting analysis using lps-stimulated thp-1 cells indicated that the inhibitory effect of iguratimod on il-1 beta production is caused by the suppression of il-1 beta mrna expression [1].
in vivo
administration of iguratimod did not inhibit the tumor growth, but resulted in attenuation of cachexia symptoms. furthermore, iguratimod decreased the serum levels of il-6, and also reduced its gene expression in the tumor tissues. in addition, exogenously administered il-6 nullified the suppressive effect of iguratimod [2].
Adverse reactions
Adverse effects include elevated transaminases, nausea, vomiting, stomach pain; rashes, and itchiness.
References
[1] tanaka k, aikawa y, kawasaki h, asaoka k, inaba t, yoshida c. pharmacological studies on 3-formylamino-7-methylsulfonylamino-6-phenoxy-4h-1-benzopyran-4-one (t-614), a novel antiinflammatory agent. 4th communication: inhibitory effect on the production of interleukin-1 and interleukin-6. j pharmacobiodyn. 1992;15(11):649-55.
[2] tanaka k, urata n, mikami m, ogasawara m, matsunaga t, terashima n, suzuki h. effect of iguratimod and other anti-rheumatic drugs on adenocarcinoma colon 26-induced cachexia in mice. inflamm res. 2007;56(1):17-23.
[3] hara m, abe t, sugawara s, mizushima y, hoshi k, irimajiri s, hashimoto h, yoshino s, matsui n, nobunaga m. long-term safety study of iguratimod in patients with rheumatoid arthritis. mod rheumatol. 2007;17(1):10-6.
Properties of Iguratimod
| Melting point: | 238.0 to 242.0 °C |
| Boiling point: | 580.6±60.0 °C(Predicted) |
| Density | 1.52±0.1 g/cm3(Predicted) |
| storage temp. | under inert gas (nitrogen or Argon) at 2-8°C |
| solubility | DMSO (Slightly) |
| form | Solid |
| pka | 5.58±0.20(Predicted) |
| color | White to Off-White |
Safety information for Iguratimod
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H361:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. |
Computed Descriptors for Iguratimod
Iguratimod manufacturer
Honour Lab Limited
SETV ASRV LLP
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 123663-49-0 IGURATIMOD 99%View Details
123663-49-0 IGURATIMOD 99%View Details
123663-49-0 -
 IGURATIMOD 98%View Details
IGURATIMOD 98%View Details -
 IGURATIMOD 123663-49-0 95-99%View Details
IGURATIMOD 123663-49-0 95-99%View Details
123663-49-0 -
 Iguratimod 97%View Details
Iguratimod 97%View Details -
 Iguratimod 123663-49-0 98%View Details
Iguratimod 123663-49-0 98%View Details
123663-49-0 -
 Iguratimod 98%View Details
Iguratimod 98%View Details
123663-49-0 -
 Iguratimod CAS 123663-49-0View Details
Iguratimod CAS 123663-49-0View Details
123663-49-0 -
 Iguratimod CAS 123663-49-0View Details
Iguratimod CAS 123663-49-0View Details
123663-49-0