Palladium(II) oxide
Synonym(s):Palladium monoxide;Palladium oxide;Palladium(II) oxide (85% Pd)
- CAS NO.:1314-08-5
- Empirical Formula: OPd
- Molecular Weight: 122.42
- MDL number: MFCD00011172
- EINECS: 215-218-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-06-17 20:04:47
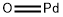
What is Palladium(II) oxide?
Chemical properties
grey to black powder
Physical properties
Greenish black tetragonal crystals; density 8.3 g/cm3; decomposes to Pd metal at 750°C; insoluble in water and acids; slightly soluble in aqua regia.
The Uses of Palladium(II) oxide
Palladium(II) oxide is used to prepare palladium catalyst for hydrogenation. The oxide is readily reduced by hydrogen to metal.
The Uses of Palladium(II) oxide
Reduction catalyst in synthesis of organic Compounds.
The Uses of Palladium(II) oxide
Catalytic reduction, in organic synthesis.Palladium(II) oxide is used as a catalyst for reduction in organic synthesis. It is an excellent gas sensing material for carbon monoxide at low working temperatures. It is also used in the preparation of good optical quality films. It is involved in the preparation of LuPd2O4 , which is used in superconducting materials.
What are the applications of Application
Palladium(II) oxide is an inorganic catalyst useful for catalytic hydrogenation
Preparation
Palladium(II) oxide is prepared by heating palladium sponge in oxygen at 350°C. The oxide is obtained as a black powder. The oxide also may be prepared specially for catalytic use by heating a mixture of palladium chloride and potassium nitrate at 600°C and then leaching out water-soluble residue. A hydrated form of the oxide, which is acid soluble can be prepared by precipitation from solution, for example, by hydrolysis of palladium nitrate. The brown hydrated oxide converts to black anhydrous oxide on heating. Its solubility in acids decreases with lowering of water content.
Flammability and Explosibility
Not classified
Properties of Palladium(II) oxide
| Melting point: | 870 °C(lit.) |
| Density | 8.7 g/mL at 25 °C(lit.) |
| RTECS | RT3592900 |
| storage temp. | Store below +30°C. |
| solubility | insoluble in H2O, acid solutions; slightly soluble in aquaregia |
| form | Viscous Liquid |
| Specific Gravity | 8.7 |
| color | Gray to black |
| Odor | Odorless |
| Water Solubility | practically insoluble |
| Merck | 14,6993 |
| CAS DataBase Reference | 1314-08-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Palladium oxide(1314-08-5) |
| EPA Substance Registry System | Palladium oxide (PdO) (1314-08-5) |
Safety information for Palladium(II) oxide
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P220:Keep/Store away from clothing/…/combustible materials. P280:Wear protective gloves/protective clothing/eye protection/face protection. P370+P378:In case of fire: Use … for extinction. P501:Dispose of contents/container to..… |
Computed Descriptors for Palladium(II) oxide
| InChIKey | HBEQXAKJSGXAIQ-UHFFFAOYSA-N |
Palladium(II) oxide manufacturer
Vineeth Precious Catalysts Pvt. Ltd.
New Products
BOC-L-4-HYDROXYPROLINE 2-nitro 3-hydroxy pyridine 2,6-Dichloropyridin-4-amine 2,3 Diamino pyridine 5-Iodo-2-(1-methylethyl)-3(2H)-pyridazinone 1-Azetidinecarboxylic acid, 3-[(3S)-1-(trans-3-carboxy-3-methylcyclobutyl)-3-piperidinyl]-, 1-(1,1-dimethylethyl) ester Tert-Butyl N-[3-(dimethylcarbamoyl)prop-2-en-1-yl]carbamate 1-(difluoromethyl)-N-methylcyclobutan-1-amine 2-(4-Methyl-1,2,5-oxadiazol-3-yl)-1H-benzimidazole 6-(4-iodophenyl)-1-oxa-6-azaspiro[3.3]he Trimethyl(phenylthio)silane Polycaprolactone(2000)-PEG(20000)-Polycaprolactone(2000) Diacrylate Diethylene Glycol Monoethyl Ether, PolyoxyethyleneOleylCetylEtherSulfosuccinate Ascorbyl Tetraisopalmitate or Tetrahexyldecyl Ascorbate Castor Oil, Ethoxylated, Cremophor EL or PEG-35 Castor Oil Tween 20 or Polysorbate 20 Acetone-d6 (R)-2-Mercaptobutanoic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine 3-(naphthalen-1-ylsulfonyl)-1H-indazol-5-amine methyl 5-amino-3-(1,1-dioxidotetrahydro-2H-1,2-thiazin-2-yl)-2-fluorobenzoate 7-methoxy-8-(2-morpholinoethoxy)-4-((3,4,5-trimethoxyphenyl)amino)benzo[g]quinoline-3-carbonitrile Dimethylaluminum isopropoxideRelated products of tetrahydrofuran

You may like
-
 1314-08-5 PALLADIUM OXIDE 99%View Details
1314-08-5 PALLADIUM OXIDE 99%View Details
1314-08-5 -
 Palladium(II) oxide 99%View Details
Palladium(II) oxide 99%View Details -
 Palladium(II) oxide, Anhydrous CAS 1314-08-5View Details
Palladium(II) oxide, Anhydrous CAS 1314-08-5View Details
1314-08-5 -
 Palladium(II) oxide, Anhydrous CAS 1314-08-5View Details
Palladium(II) oxide, Anhydrous CAS 1314-08-5View Details
1314-08-5 -
 Palladium oxide CAS 1314-08-5View Details
Palladium oxide CAS 1314-08-5View Details
1314-08-5 -
 Palladium oxide, ≥99.97% CAS 1314-08-5View Details
Palladium oxide, ≥99.97% CAS 1314-08-5View Details
1314-08-5 -
 Palladium oxide, ≥99% CAS 1314-08-5View Details
Palladium oxide, ≥99% CAS 1314-08-5View Details
1314-08-5 -
 PALLADIUM (II) OXIDE HYDRATE Extra Pure CAS 1314-08-5View Details
PALLADIUM (II) OXIDE HYDRATE Extra Pure CAS 1314-08-5View Details
1314-08-5
