Neodymium oxide
Synonym(s):Dineodymium trioxide;Neodymia;Neodymium sesquioxide;Neodymium(III) oxide
- CAS NO.:1313-97-9
- Empirical Formula: Nd2O3
- Molecular Weight: 336.48
- MDL number: MFCD00011134
- EINECS: 215-214-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-22 18:04:34
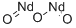
What is Neodymium oxide?
Chemical properties
Neodymium Oxide, Nd2O3 is a blue-grey powder that is composed of the rare earth neodymium and oxygen. hygroscopic; absorbs atmospheric CO2; hexagonal; has slightly red fluorescence. Water insoluble, soluble in acids. It has special properties that make it very useful to the glass and ceramics industries. Neodymium Oxide gives a good aqua color in most glaze bases at 1-2%. At 4-7% it gives a pleasant lavender/grape color that appears as a different color under different artificial light sources. It is very strongly affected by iron, particularly in oxidation, giving a reliable perfect neutral transparent gray. Avoid trace iron impurities to achieve the brightest blue and lavender colors.
Physical properties
Blue powder; hexagonal crystals; fluoresces red; density 7.24 g/cm3; melts around 1,900°C; practically insoluble in water, 30 mg/L at 75°C; dissolves in acids.
The Uses of Neodymium oxide
Neodymium(III) oxide is an inorganic compound containing inner transition metal neodymium from lanthanides series in +3 oxidation state and oxide in 2:3 ratio. It adopts a hexagonal crystal structure. It is also known as neodymium oxide, neodymiumtrioxide, neodymium(3+)oxide, neodymium sesquioxide and dineodymiumtrioxide. It used as ceramic capacitors, coloring glass, carbon arc-light electrodes, color TV tubes, dehydrogenation catalyst, (65%) To counteract color of iron in glass.
The Uses of Neodymium oxide
Neodymium oxide (Nd2O3) is a light-blue powder used to color glass and as a pigment for ceramics. It is also used to make color TV tubes.
Production Methods
Neodymium oxide is produced from the two principal rare earth minerals, monazite, and bastnasite. The oxide is obtained as an intermediate in the recovery of neodymium metal (See Neodymium).
The oxide also may be formed by thermal dissociation of neodymium oxalate, hydroxide or carbonate:
Nd2(C2O4)3 → Nd2O3 + 6CO2
2Nd(OH)3 → Nd2O3 + 3H2O
Nd2(CO3)3 → Nd2O3 + 3CO2.
Reactions
The anhydrous oxide absorbs moisture from the air at ambient temperatures forming hydrated oxide. The oxide also absorbs carbon dioxide from air, forming neodymium carbonate.
Neodymium oxide dissolves in strong mineral acids forming corresponding neodymium salts:
Nd2O3 + 3H2SO4 → Nd2(SO4)3 + 3H2O
Reactions with acetic and other organic acids produce corresponding salts. When heated with ammonium chloride at 300 to 400°C, the oxide converts to chloride liberating ammonia and water:
Nd2O3 + 6NH4Cl → 2NdCl3 + 6NH3 + 3H2O
When heated with hydrogen fluoride, the product is neodymium fluoride:
Nd2O3 + 6HF → 2NdF3 + 3H2O
The oxide is reduced to neodymium metal when heated with hydrogen, carbon monoxide, or other reducing agents.
Flammability and Explosibility
Non flammable
Toxicology
Neodymium oxide is belong to a rare earth metal. These metals are moderately to highly toxic. The symptoms of toxicity of the rare earth elements include writhing, ataxia, labored respiration, walking on toes with arched back and sedation.
Safety Profile
Low toxicity by ingestion.
Purification Methods
Dissolve it in HClO4, precipitate it as the oxalate with doubly recrystallised oxalic acid, wash it free of soluble impurities, dry it at room temperature and ignite it in a platinum crucible at higher than 850o in a stream of oxygen. It is a blue powder. [Tobias & Garrett J Am Chem Soc 80 3532 1958.]
References
https://en.wikipedia.org/wiki/Neodymium#Neodymium_glass_for_other_applications
https://en.wikipedia.org/wiki/Neodymium(III)_oxide
https://books.google.kg/books?id=KbZkxDyeG18C&pg=PA102&dq=%22Neodymium+oxide%22&redir_esc=y#v=onepage&q=%22Neodymium%20oxide%22&f=false
Properties of Neodymium oxide
| Melting point: | 2270 °C |
| Boiling point: | 3760℃[at 101 325 Pa] |
| Density | 7.24 g/mL at 20 °C(lit.) |
| storage temp. | no restrictions. |
| solubility | insoluble in H2O; soluble in dilute acid solutions |
| form | Sintered Lump |
| color | Yellow |
| Specific Gravity | 7.24 |
| Water Solubility | insoluble |
| Merck | 14,6450 |
| CAS DataBase Reference | 1313-97-9(CAS DataBase Reference) |
| EPA Substance Registry System | Neodymium oxide (Nd2O3) (1313-97-9) |
Safety information for Neodymium oxide
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Neodymium oxide
Neodymium oxide manufacturer
Star Earth Minerals Pvt Ltd
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Neodymium(III) oxide CAS 1313-97-9View Details
Neodymium(III) oxide CAS 1313-97-9View Details
1313-97-9 -
 Neodymium(III) oxide CAS 1313-97-9View Details
Neodymium(III) oxide CAS 1313-97-9View Details
1313-97-9 -
 Neodymium(III) oxide CAS 1313-97-9View Details
Neodymium(III) oxide CAS 1313-97-9View Details
1313-97-9 -
 Neodymium(III) oxide CAS 1313-97-9View Details
Neodymium(III) oxide CAS 1313-97-9View Details
1313-97-9 -
 Neodymium(III) oxide CAS 1313-97-9View Details
Neodymium(III) oxide CAS 1313-97-9View Details
1313-97-9 -
 Neodymium(III) oxide CAS 1313-97-9View Details
Neodymium(III) oxide CAS 1313-97-9View Details
1313-97-9 -
 Neodymium(III) oxide CAS 1313-97-9View Details
Neodymium(III) oxide CAS 1313-97-9View Details
1313-97-9 -
 Neodymium Oxide CASView Details
Neodymium Oxide CASView Details