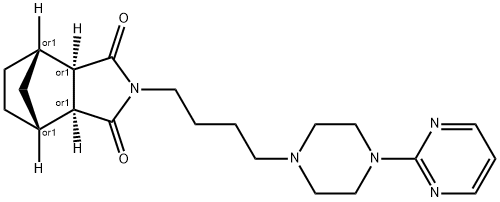
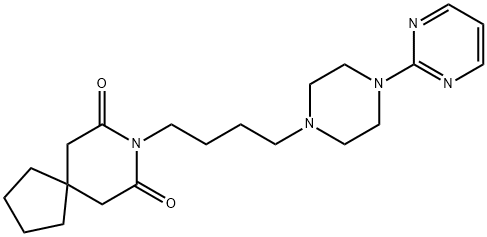
Tandospirone
Synonym(s):(1R*,2S*,3R*,4S*)-N-(4-(4-(2-Pyrimidinyl)-1-piperazinyl)butyl)-2,3-norbornanedicarboximide citrate salt;SM 3997
- CAS NO.:87760-53-0
- Empirical Formula: C21H29N5O2
- Molecular Weight: 383.49
- MDL number: MFCD00904852
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-28 23:16:16
What is Tandospirone?
Description
Tandospirone is another 5-HT1A receptor agonist under development by Sumitomo Pharmaceutical Co., Ltd., in Japan and Pfizer in the United States. Results of phase II and phase III clinical trials show that tandospirone is effective for the treatment of anxiety neurosis. Significant improvement is also observed in patients with psychosomatic disease, phobia, and depersonalization (Murasaki 1995). The results have shown that initial treatment should be started at 30 mg daily, and the dose should be increased up to 60 mg daily according to symptoms (Murasaki 1995). A double-blind comparative study with diazepam revealed that tandospirone tends to be superior to diazepam in patients with depressive neurosis, whereas diazepam may be more effective than tandospirone in severely ill patients (Murasaki et al. 1992).
Originator
Sumitomo (Japan)
The Uses of Tandospirone
Tandospirone is a 5HT1A receptor partial agonist. Studies indicate that tandospirone significantly reduces haloperidol-induced bradykinesia in a dose dependent manner. The potency of Tandospirone is equal to that of buspirone and approximate one-half that of diazepam. The potency of Tandospirone at dopamine antagonistic action is less than 1/4 that of buspirone.
Preparation
Tandospirone is attained from the exo diels-alder adduct of maleic anhydride and cyclopentadiene in a four step convergent approach.
Definition
ChEBI: Tandospirone is a dicarboximide that is (3aR,4S,7R,7aS)-hexahydro-1H-4,7-methanoisoindole-1,3(2H)-dione which is substituted by a 4-[4-(pyrimidin-2-yl)piperazin-1-yl]butyl group at position 2. It is a potent and selective 5-HT1A receptor partial agonist (Ki = 27 nM). It has a role as an antidepressant and an anxiolytic drug. It is a N-alkylpiperazine, a N-arylpiperazine, a member of pyrimidines, a bridged compound and a dicarboximide. It is a conjugate base of a tandospirone(1+).
brand name
Sediel
Biological Activity
5-HT 1A receptor partial agonist (K i = 27 nM) that displays selectivity over 5-HT 2 , 5-HT 1C , α 1 , α 2 , D 1 and D 2 receptors (K i values ranging from 1300-41000 nM). Inactive at 5-HT uptake sites, 5-HT 1B , β -adrenergic, muscarinic and benzodiazepine receptors. Displays anxiolytic activity.
References
[1] shimizu h1, hirose a, tatsuno t, nakamura m, katsube j. pharmacological properties of sm-3997: a new anxioselective anxiolytic candidate. jpn j pharmacol. 1987 dec; 45(4):493-500.
Properties of Tandospirone
| Melting point: | 112-113.5° |
| Boiling point: | 613.9±65.0 °C(Predicted) |
| Density | 1.239±0.06 g/cm3(Predicted) |
| storage temp. | Store at +4°C |
| solubility | DMSO: soluble38mg/mL |
| pka | 7.71±0.10(Predicted) |
| form | Solid |
| color | White to Off-White |
Safety information for Tandospirone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Tandospirone
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
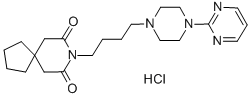
 Tandospirone CAS 87760-53-0View Details
Tandospirone CAS 87760-53-0View Details
87760-53-0 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1