Buspirone hydrochloride
Synonym(s):Buspirone hydrochloride;N-[4-[4-(2-Pyrimidinyl)-1-piperazinyl]butyl]-8-azaspiro[4.5]decane-7,9-dione hydrochloride
- CAS NO.:33386-08-2
- Empirical Formula: C21H32ClN5O2
- Molecular Weight: 421.96
- MDL number: MFCD00078569
- EINECS: 251-489-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
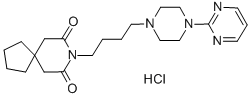
What is Buspirone hydrochloride?
Description
Buspirone hydrochloride is an anxiolytic agent indicated for the management of anxiety disorders with or without accompanying depression. In contrast to the benzodiazepines, buspirone does not interact with alcohol, and lacks sedative, anticonvulsant, and muscle relaxant effects, and abuse potential.
Chemical properties
White Solid
Originator
Mead Johnson (USA)
The Uses of Buspirone hydrochloride
Non-benzodiazepine anxiolitic; 5-hydroxytryptamine (5-HT1) receptor agonist.
The Uses of Buspirone hydrochloride
Non-benzodiazepine anxiolytic; 5-hydroxytryptamine (5-HT1) receptor agonist.
The Uses of Buspirone hydrochloride
anxiolitic;serotonin receptor agonist
What are the applications of Application
Buspirone hydrochloride is a SR-1A agonist
Definition
ChEBI: A hydrochloride salt resulting from the reaction of equimolar amounts of buspirone and hydrogen chloride.
Manufacturing Process
There is the 3 methods for preparing of 8-azaspiro(4.5)decane-7,9-dione, 8-
(4-(4-(2-pyrimidinyl)-1-piperazinyl)butyl) monohydrochloride (U.S. Patent
3,717,634). One of them is follows: a mixture of 0.1 mole of the substituted
glutaric anhydride, 0.1 mole of l-(4-aminobutyl)-4-(2-pyrimidinyl)piperazine
(U.S. Pat. 3,398151), and 300 ml of pyridine was refluxed until imide
formation was completed. The degree of reaction was readily followed by
taking an aliquot portion of the reaction mixture, removing the solvent, and
obtaining the infrared absorption spectrum of the residue. When reaction is
complete, the spectrum exhibited typical infrared imide bands at 1701 and
1710 cm-1 whereas if incomplete, the infrared spectrum contains amide and
carboxyl absorption bands at 1680, 1760 and 3300 cm-1.
1-(3-Cyanopropyl)-4-(2-pyrimidinyl)-piperazine. A mixture of 1-(2-
pyrimidinyl)piperazine (6.0 g, 0.04 mole), 4.6 g (0.044 mole) of 3-
chloropropionitrile and sodium carbonate (4.24 g, 0.04 mole) in 50 ml of nbutanol
was gently refluxed for 16 hours. The reaction mixture was
concentrated in vacuo and the residual oil dissolved in about 100 ml of
cyclohexane. On standing a white crystalline material separated which was
crystallized from cyclohexane to provide 6.5 g (yield 70%) of the cyano intermediate, m.p. 56.6-58°C. A solution of 11.5 g (0.05 mole) of 1-(3-
cyanopropyl)-4-(2-pyrimidinyl)piperazine in 150 ml of absolute ethanol was
saturated with ammonia. W-6 Raney nickel catalyst was added and the
mixture hydrogenated under 1200 p.s.i. When the hydrogenation was
completed the mixture was filtered and the residual oil distilled under reduced
pressure to provide 8.2 g (70% ) of 1-(4-aminobutyl)-4-(2-
pyrimidinyl)piperazine, b.p. 143-146°C at 0.1 mm. (nD
26 = 1.5582).
The azospiroalkenedione 8-[4-[4-(2-pyrimidinyl)-1-piperazinyl]butyl]-8-
azaspiro[4.5]decane-7,9-dione was purified as free base by stripping off the
pyridine solvent and crystallizing the residue from a suitable solvent or by
vacuum distillation thereof hydrochloric salt of it was prepared by treating of
an ethanol solution of free base with equimolar amount of HCl.
brand name
Buspar (Bristol-Myers Squibb);BESPAR.
Therapeutic Function
Anxiolytic
General Description
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards
Biological Activity
Classic 5-HT 1A partial agonist with relatively high affinity (K i = 9.3 - 29.5 nM). A clinically effective anxiolytic.
Biochem/physiol Actions
5-HT1A serotonin receptor agonist; anxiolytic.
Clinical Use
Anxiolytic
Veterinary Drugs and Treatments
Buspirone may be effective in treating certain behavior disorders in dogs and cats, principally those that are fear/phobia related and especially those associated with social interactions. Buspirone may also be useful for urine spraying or treatment of motion sickness in cats.
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: concentration increased by
erythromycin - reduce dose; concentration reduced
by rifampicin.
Antidepressants: avoid with tranylcypromine; risk of
severe hypertension with MAOIs - avoid.
Antifungals: concentration increased by itraconazole
- reduce dose.
Antipsychotics: enhanced sedative effects;
haloperidol concentration increased.
Antivirals: concentration increased by ritonavir,
increased risk of toxicity.
Calcium-channel blockers: concentration increased
by diltiazem and verapamil - reduce dose.
Grapefruit juice: concentration increased by
grapefruit juice - reduce dose.
Methylthioninium: possible risk of CNS toxicity -
avoid if possible.
Metabolism
Systemic bioavailability of buspirone is low because of extensive first-pass metabolism. Metabolism in the liver is extensive via the cytochrome P450 isoenzyme CYP3A4; hydroxylation yields several inactive metabolites and oxidative dealkylation produces 1-(2-pyrimidinyl)- piperazine, which is reported to be about 25% as potent as the parent drug in one model of anxiolytic activity. Buspirone is excreted mainly as metabolites in the urine, and also the faeces.
storage
+4°C (desiccate)
Properties of Buspirone hydrochloride
| Melting point: | 201.5-202.50C |
| Flash point: | 9℃ |
| storage temp. | 2-8°C |
| solubility | Freely soluble in water and in methanol, practically insoluble in acetone. |
| form | neat |
| form | Solid |
| color | White to off-white |
| Water Solubility | Soluble in methanol (50 mg/ml), water (partly), DMSO (100 mM). |
| CAS DataBase Reference | 33386-08-2(CAS DataBase Reference) |
Safety information for Buspirone hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Buspirone hydrochloride
Buspirone hydrochloride manufacturer
Medilux Laboratories Pvt Ltd
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
![33386-08-2 Buspirone Hydrochloride
8-[4-[4-(Pyrimidin-2-yl)piperazin-1-yl]butyl]-8-azaspiro[4.5]- decane-7,9-dione hydrochloride 96%](https://img.chemicalbook.in//ProductImageIndia/2024-03/Raw/a0c92169-e5fb-4435-96a5-907a82caf324.png) 33386-08-2 Buspirone Hydrochloride 8-[4-[4-(Pyrimidin-2-yl)piperazin-1-yl]butyl]-8-azaspiro[4.5]- decane-7,9-dione hydrochloride 96%View Details
33386-08-2 Buspirone Hydrochloride 8-[4-[4-(Pyrimidin-2-yl)piperazin-1-yl]butyl]-8-azaspiro[4.5]- decane-7,9-dione hydrochloride 96%View Details
33386-08-2 -
 33386-08-2 98%View Details
33386-08-2 98%View Details
33386-08-2 -
 Buspirone hydrochloride 98%View Details
Buspirone hydrochloride 98%View Details
33386-08-2 -
 33386-08-2 99%View Details
33386-08-2 99%View Details
33386-08-2 -
 Buspirone hydrochloride CAS 33386-08-2View Details
Buspirone hydrochloride CAS 33386-08-2View Details
33386-08-2 -
 Buspirone HCl 99% (HPLC) CAS 33386-08-2View Details
Buspirone HCl 99% (HPLC) CAS 33386-08-2View Details
33386-08-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8