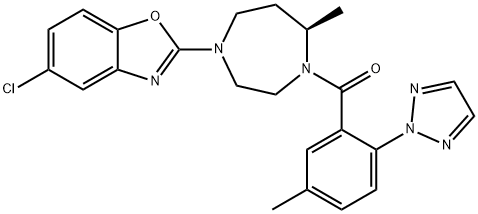
Suvorexant
- CAS NO.:1030377-33-3
- Empirical Formula: C23H23ClN6O2
- Molecular Weight: 450.92
- MDL number: MFCD22377755
- EINECS: 685-109-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-02-19 08:55:38
What is Suvorexant?
Absorption
Peak concentrations occur at a median Tmax of 2 hours under fasted conditions. Ingestion of suvorexant with a high-fat meal has no effect on AUC or Cmax, but may delay Tmax by approximately 1.5 hours. Mean absolute bioavailability of 10 mg is 82%.
Toxicity
Dose-related somnolence and CNS depression are the most common adverse effects associated with the use of suvorexant. It has also been shown to impair driving skills and may increase the risk of falling asleep while driving. Next-day impairments are found to be highest if suvorexant is taken with less than a full night of sleep remaining, with higher doses, or if co-administered with other CNS depressants or CYP3A inhibitors. Complex behaviours such as sleep driving, preparing and eating food, and making phone calls have been reported in association with the use of hypnotics such as suvorexant. A dose-dependant increase in suicidal ideation has been observed, especially in patients with a previous diagnosis of depression. Sleep paralysis, hypnagogic/hypnopompic hallucinations including vivid and disturbing perceptions, and mild cataplexy have also been reported. There are no adequate studies in pregnant women to ensure its safety during pregnancy or breast feeding.
Description
Suvorexant, a dual orexin receptor antagonist marketed under the trade name Belsomra®, discovered and developed by Merck for the treatment of insomnia, was approved by the US FDA in August 2014 and became available in Japan in November of the same year. The drug’s mechanism of action operates through the competitive blockade of wake-promoting neuropeptides orexin A and orexin B toward receptors orexin receptor type 1 and orexin receptor type 2, which are believed to modulate sleep-wake cycles.
The Uses of Suvorexant
Suvorexant (MK-4305) is a dual (non-selective) orexin receptor antagonist in development by Merck & Co. for the treatment of insomnia. It works by turning off wakefulness rather than by inducing sleep. Users of higher doses had an increased rate of suicidal ideation.
Indications
Suvorexant is indicated for the treatment of insomnia characterized by difficulties with sleep onset and/or sleep maintenance.
Background
Suvorexant is a selective dual antagonist of orexin receptors OX1R and OX2R that promotes sleep by reducing wakefulness and arousal. It has been approved for the treatment of insomnia.
Definition
ChEBI: Suvorexant is an aromatic amide obtained by formal condensation of the carboxy group of 5-methyl-2-(2H-1,2,3-triazol-2-yl)benzoic acid with the secondary amino group of 5-chloro-2-[(5R)-5-methyl-1,4-diazepan-1-yl]-1,3-benzoxazole. An orexin receptor antagonist used for the management of insomnia. It has a role as a central nervous system depressant and an orexin receptor antagonist. It is a member of 1,3-benzoxazoles, a member of triazoles, a diazepine, an aromatic amide and an organochlorine compound.
Biological Activity
Suvorexant is a dual orexin receptor (OXR) antagonist that blocks both OX1R and OX2R (Kis = 1.2 and 0.60 nM, respectively). It reduces locomotor activity and promotes sleep by inhibiting the binding of orexin A and B. In rats, suvorexant decreases self-administration of, and conditioned place preference for, cocaine ( | 16186 | ISO60176). It also decreases dopamine levels in the rat ventral striatum following a cocaine-induced increase. Formulations containing suvorexant are used in the treatment of insomnia. Suvorexant is regulated as a Schedule IV compound in the United States. This compound is also available as an analytical reference standard.
Synthesis
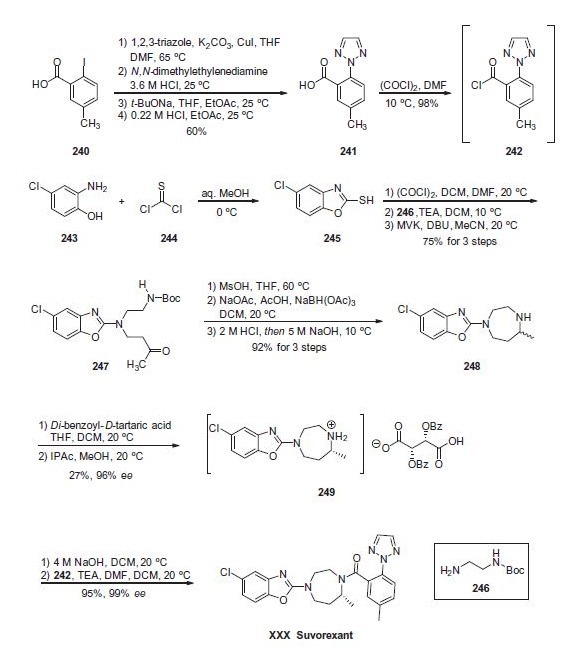
Commercially available acid 240 was first
subjected to a copper-assisted substitution reaction involving
1,2,3-triazole in DMF at elevated temperatures. Although these
conditions resulted in an excellent yield of a triazole-substituted
product, an approximate 4:1 ratio of the desired 2-arylated triazole
241 and the undesired 1-arylated triazole byproduct were recovered
from the reaction. The mixture was then treated with N,Ndimethylethylenediamine
in acid to sequester copper. Next, the
mixture of arylated triazoles was carefully subjected to sodium tbutoxide
in DMF and ethyl acetate to form the corresponding
sodium salts, and interestingly it was found that the desired
sodium salt of 241 could be isolated based on its solubility profile
under these conditions. Acidification of the desired carboxylate salt
using dilute HCl gave rise to acid 241 in 60% yield across the fourstep
sequence. Next, subjection of this acid to oxalyl chloride in
chilled DMF generated the acid chloride 242 in excellent yield. This
crude acid chloride was used immediately in the next step of the
synthetic sequence.
Metabolism
Suvorexant is primarily metabolized by cytochrome-P450 3A4 enzyme (CYP3A4) with a minor contribution from CYP2C19. Major circulating metabolites are suvorexant and a hydroxy-suvorexant metabolite, which is not expected to be pharmacologically active. There is potential for drug-drug interactions with drugs that inhibit or induce CYP3A4 activity.
Metabolism
Suvorexant is primarily metabolized by cytochrome-P450 3A4 enzyme (CYP3A4) with a minor contribution from CYP2C19. Major circulating metabolites are suvorexant and a hydroxy-suvorexant metabolite, which is not expected to be pharmacologically active. There is potential for drug-drug interactions with drugs that inhibit or induce CYP3A4 activity.
Properties of Suvorexant
| Melting point: | 153℃ |
| Boiling point: | 669.8±65.0 °C(Predicted) |
| Density | 1.41 |
| storage temp. | Room Temperature |
| solubility | Acetonitrile (Slightly), Chloroform (Slightly), DMSO (Slightly, Heated), Methano |
| form | Solid |
| pka | 1.47±0.40(Predicted) |
| color | White to Pale Beige |
Safety information for Suvorexant
Computed Descriptors for Suvorexant
| InChIKey | JYTNQNCOQXFQPK-MRXNPFEDSA-N |
| SMILES | C(N1[C@H](C)CCN(C2=NC3=CC(Cl)=CC=C3O2)CC1)(C1=CC(C)=CC=C1N1N=CC=N1)=O |
Abamectin manufacturer
SHOBHA LIFE SCIENCES PRIVATE LIMITED
Mehta API Pvt. Ltd.
Ralington Pharma
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 1030377-33-3 Suvorexant 99%View Details
1030377-33-3 Suvorexant 99%View Details
1030377-33-3 -
 1030377-33-3 98%View Details
1030377-33-3 98%View Details
1030377-33-3 -
 Suvorexant 98%View Details
Suvorexant 98%View Details -
 Suvorexant 1030377-33-3 98%View Details
Suvorexant 1030377-33-3 98%View Details
1030377-33-3 -
 1030377-33-3 Suvorexant 98%View Details
1030377-33-3 Suvorexant 98%View Details
1030377-33-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4