Ethylene
Synonym(s):Ethene
- CAS NO.:74-85-1
- Empirical Formula: C2H4
- Molecular Weight: 28.05
- MDL number: MFCD00008604
- EINECS: 200-815-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
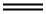
What is Ethylene?
Description
Ethylene is a colorless, odorless gas that is the simplest alkene hydrocarbon. It is a natural plant hormone and is produced synthetically from natural gas and petroleum. The double bond in ethylene makes this compound highly reactive, and the volume of ethylene used in the chemical industry is greater than any other organic compound.
Description
Ethylene, the simplest olefin, is believed to have been discovered by J. J. Becher ca. 1669. He prepared it by heating ethanol with sulfuric acid, but in modern times most ethylene is produced by steam cracking light hydrocarbons.
We know ethylene as the raw material for polyethylene, but it also plays a significant role in plant biology. Ethylene "tells" leaves when to change colors, wilt, and drop. It stimulates flowers to open and wither, and fruit to ripen. Many fruit growers make use of these natural processes by exposing their crops to ethylene to control flowering and fruiting or to make harvesting easier.
Chemical properties
colourless gas
Chemical properties
Ethylene, an alkene is a colorless gas (at room temperature). Sweet odor. Minimum detectable odor 5 260 ppm.
The Uses of Ethylene
Oxyethylene welding and cutting metals; manufacture of alcohol, mustard gas, and many other organics. manufacture of ethylene oxide (for plastics), "Polythene", polystyrene and other plastics. Plant growth regulator; used commercially to accelerate the ripening of various fruits.
The Uses of Ethylene
Ethylene occurs in petroleum gases, in illuminatinggas, and in ripening fruits. It is made by dehydrating alcohol. It is used in oxyethyleneflame for welding and cutting metals; inthe manufacture of polyethylene, polystyrene,and other plastics; in making ethylene oxide;and as an inhalation anesthetic.
The Uses of Ethylene
Ethylene is highly reactive and is one of the most important compounds for the chemical industry. The highest use of ethylene is in polymerization reactions. Polyethylene polymers are linear, but they contain side branchings of methyl groups. Among these are several groups defi ned by their density produced under different pressure regimens.
The second highest use of ethylene involves oxidation to ethylene oxide and its derivative ethylene glycol (HO-CH2-CH2-OH). Ethylene glycol is used mainly as antifreeze and in the production of polyesters. Other important compounds produced through oxidation of ethylene are acetaldehyde (H3C-CH = O) and vinyl acetate (CH2 = CH-O-CO-CH2). Ethylene may also be halogenated to produce a number of other compounds.
Definition
ethylene: A colourlessflammable gaseous hydrocarbon,C2H4; m.p. –169°C; b.p. –103.7°C. It isthe first member of the alkene seriesof hydrocarbons. It is made bycracking hydrocarbons from petroleumand is now a major raw materialfor making other organicchemicals (e.g. ethanal, ethanol,ethane-1,2-diol). It can be polymerizedto polyethene. It occurs naturallyin plants, in which it acts as agrowth substance promoting theripening of fruits.
Production Methods
Ethylene is primarily obtained from the ethane and propane components of natural gas andfrom the naphtha, kerosene, and gas oil components of crude oil. It can also be synthesizedthrough the dehydration of ethanol (C2H5OH). The production of ethylene from hydrocarbonfeedstocks involves mixing with steam and then subjecting the hydrocarbons to thermalor catalytic cracking. Cracking is a process in which organic molecules are broken down intosmaller molecules. Thermal cracking involves the use of heat and pressure. Catalytic crackinguses various catalysts to reduce the amount of heat and pressure required in the process.Th ermal cracking of hydrocarbons to ethylene occurs between approximately 650°C and800°C (1200°F and 1500°F). After hydrocarbons are cracked, a mixture containing ethyleneand other gases such as methane, ethane, and propane is obtained. Ethylene is separated fromthese through physical processes such as fractional distillation, refrigeration, absorption, oradsorption.
Reactions
Ethylene reacts: (1) with the halogens to form substitution halides; (2) with hypochlorous and hypobromous acid to form ethylene chlorohydrin or ethylene bromohydrin, respectively; (3) with hydrogen iodide or bromide (not chloride) to form ethyl iodide or ethyl bromide; (4) with hydrogen, in the presence of a catalyst, e.g., finely divided nickel at 150 °C, to form ethane; (5) with concentrated sulfuric acid at 160 °C to form ethyl hydrogen sulfate; and (6) with potassium permanganate to form ethylene glycol, although glycol is preferably made from ethylene dichloride or chlorohydrin.
General Description
A colorless gas with a sweet odor and taste. ETHYLENE is lighter than air. ETHYLENE is easily ignited and a flame can easily flash back to the source of the leak. Under prolonged exposure to fire or heat the containers may rupture violently and rocket. Can cause explosion.
Air & Water Reactions
Highly flammable.
Reactivity Profile
Peroxidizable monomer may initiate exothermic polymerization of the bulk material [Bretherick 1979. p. 160]. ETHYLENE in the presence of aluminum chloride may undergo a violent reaction [J. Inst. Pet. 33:254. 1947]. Ozone and ETHYLENE react explosively [Berichte 38:3837]. ETHYLENE can polymerize at low pressure if catalyzed by titanium halides. (Sundaram, K. M, M. M. Shreehan, E. F. Olszewski. thylene. Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc. 2001.)
Hazard
Highly flammable, dangerous fire and explosion risk; explosive limits in air 3–36% by volume. Simple asphyxiant; questionable carcinogen.
Health Hazard
Moderate concentration in air causes drowsiness, dizziness, and unconsciousness. Overexposure causes headache, drowsiness, muscular weakness.
Health Hazard
Exposure to ethylene atmosphere can causeasphyxiation. At high concentrations it is anarcotic and can cause unconsciousness.
Fire Hazard
Flammable gas; burns with a luminous flame;
autoignition temperature 490°C (914°F)
(NFPA 1997), 543°C (1009°F) (Merck
1996); fire-extinguishing measure: shut off
the flow of gas; use a water spray to keep
fire-exposed containers cool.
Ethylene forms explosive mixtures in
air; the LEL and UEL values are 2.7%
and 36% by volume of air, respectively.
Its reaction with fluorine is explosively
violent (△H=- 112 kcal/mol), and violent
with chlorine (△H=- 36 kcal/mol). In the
presence of sunlight or UV light, an ethylene–
chlorine mixture will explode spontaneously.
The reaction is explosive at room
temperature over the oxides of mercury or
silver (Mellor 1946, Suppl. 1956). Ethylene
reacts vigorously with oxidizing substances.
It reacts with ozone to form ethylene
ozonide, H2C(O3)CH2, which is unstable
and explodes on mechanical shock. Acidcatalyzed
addition of hydrogen peroxide
may produce ethyl hydroperoxide, which is
unstable and explodes on heat or shock:.
Industrial uses
Ethylene, also called ethane, is a colorless,inflammable gas, CH2:CH2, produced in thecracking of petroleum. Ethylene liquefies at–68.2°C. Ethylene is the largest-volume organicchemical produced today, and is the basic buildingblock of the petrochemical industry. Polymerizationof ethylene is its largest use. Whenethylene is reacted in the presence of transitionmetal catalysts, such as Mo2O5 or Cr2O3, at highpressures, it forms low-density polyethylene(LDPE); at lower pressures, high-density polyethylene(HDPE) is produced. Recently, lowpressures have been employed for making a newvariant, linear low-density polyethylene(LLDPE). Ethylene is now used to produce ethylalcohol, acrylic acid, and styrene, and it is thebasis for many types of reactive chemicals.
Trichloroethylene is a colorless liquid ofpleasant odor of the composition CHCl:CCl2,also known as westrosol. Its boiling point is87°C and its specific gravity 1.471. It is insolublein water and is unattacked by dilute acidsand alkalis. It is non flammable and is less toxicthan tetrachlorethane. Trichloroethylene is apowerful solvent for fats, waxes, resins, rubber,and other organic substances, and is employedfor the extraction of oils and fats, for cleaningfabrics, and for degreasing metals preparatoryto plating. The freezing point is –88°C, and itis also used as a refrigerant. It is also used insoaps employed in the textile industry fordegreasing.
Materials Uses
Installations must be designed to withstand the pressures involved and must comply with all applicable regulations. Because it is noncorrosive, any common commercially available metals may be used with ethylene.
Safety Profile
Suspected carcinogen. A simple asphyxiant. High concentrations cause anesthesia. A common air contaminant. It is phytotoxic. A very dangerous fire hazard when exposed to heat or flame. Moderate explosion hazard when exposed to flame. A flammable gas. To figh fire, stop flow of gas, use Co2, dry chemical, or fine water spray. Mixtures with aluminum chloride explode in the presence of nickel catalysts, methyl chloride, or nitromethane. Explosive reaction with bromotrichloromethane (at 120℃/51 bar), carbon tetrachloride (25-100°C/30 bar). Explosive reaction with chlorine catalyzed by sunlight or UV light or in the presence of mercury(I) oxide, mercury(Ⅱ) oxide, or silver oxide. Mixtures with chlorotrifluoroethylene polymerize explosively when exposed to 50 kV gamma rays at 308 krad/hr. Has been involved in industrial accidents. Violent polymerization is catalyzed by copper above 4OO0C/54 bar. Incompatible with AlCl3, (CC4 + benzoyl peroxide), (bromotrichloromethane + NCh), 03, CCl4, Cl2, NOx, tetrafluoroethylene trifluorohypofluorite. When heated to decomposition it emits acrid smoke and irritating fumes
Potential Exposure
Ethylene is used in production of fabricated plastics, antifreeze; making fibers; to manufacture ethylene oxide, polyethylene for plastics, alcohol, mustard gas and other organics. It is used to accelerate ripening of fruit; as an anesthetic; and for oxyethylene welding and cutting of metals
Physiological effects
When used for anesthesia, ethylene is a nontoxic
gas found pleasant and nonirritating by
patients. Prolonged inhalation of substantial
concentrations results in unconsciousness; light
and moderate anesthesia is attained, and deep
anesthesia seldom occurs.
Inhalation is fatal only if the gas acts as a
simple asphyxiant, depriving the body of necessary
oxygen. Because of its flammability, however,
other agents have replaced ethylene for
use in anesthesia in the United States and Canada.
No deleterious action by ethylene on circulatory,
respiratory, or other systems or organs has
been observed. Exhalation eliminates the major
portion of ethylene within minutes, although
complete desaturation from body fat takes several
hours. Minute traces can be detected in the
blood a number of hours after anesthesia has
ended.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit. If frostbite has occurred, seekmedical attention immediately; do NOT rub the affectedareas or flush them with water. In order to prevent furthertissue damage, do NOT attempt to remove frozen clothingfrom frostbitten areas. If frostbite has NOT occurred,immediately and thoroughly wash contaminated skin withsoap and water.
Carcinogenicity
A case-control study of brain cancer among Texas petrochemical workers reported increased risks associated with exposure to multiple chemicals, including ethene. The findings were not statistically significant. The risks for ethene have not been shown to increase with increasing duration of employment. The observed increases could not be attributed to specific chemical exposures.
Environmental Fate
Emitted ethene is distributed primarily into the atmosphere and reacts with photochemically reactive hydroxyl radicals, ozone, and nitrate radicals, with half-lives ranging from 1.9, 6.5, and 190 days, respectively. Biodegradation in water occurs with half-lives in the range of 1–28 days, or under anaerobic conditions, 3–112 days. Bioaccumulation in aquatic organisms is not expected to occur, based on ethene’s high vapor pressure and log octanol/water partition coefficient.
Storage
Ethylene is stored in a cool, well-ventilatedarea isolated from oxygen, chlorine, andflammable and oxidizing substances. It isprotected against lightning, statical electricity,heat, and physical damage. It is shippedin steel pressure cylinders and tank barges.
Shipping
UN1962 Ethylene, Hazard Class: 2.1; Labels: 2.1-Flammable gas; UN1038 Ethylene, refrigerated liquid (cryogenic liquid), Hazard Class: 2.1; Labels: 2.1- Flammable gas. Cylinders must be transported in a secure upright position, in a well-ventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written permission of the owner.
Purification Methods
Purify ethylene by passage through a series of towers containing molecular sieves or anhydrous CaSO4 or a cuprous ammonia solution, then conc H2SO4, followed by KOH pellets. Alternatively, it has been condensed in liquid nitrogen, with melting, freezing and pumping to remove air before passage through an activated charcoal trap, followed by a further condensation in liquid air. A sputtered sodium trap was used to remove oxygen. [Beilstein 1 IV 677.]
Toxicity evaluation
Ethene is classified as a simple asphyxiant. In sufficient concentrations, ethene causes central nervous system depression and unconsciousness by displacing oxygen in air, which reduces the oxygen available to support cell function.
Incompatibilities
A highly flammable gas at room temperature. Contact with oxidizers may cause explosive polymerization and fire. May be spontaneously explosive in sunlight or ultraviolet light when mixed with chlorine. Reacts violently with mixtures of carbon tetrachloride and benzoyl peroxide; bromotrichloromethane; aluminum chloride and ozone. Incompatible with acids, halogens, nitrogen oxides; hydrogen bromide; aluminum chloride; chlorine dioxide; nitrogen dioxide. May accumulate static electrical charges, and may cause ignition of its vapors.
Waste Disposal
Return refillable compressed gas cylinders to supplier
GRADES AVAILABLE
Ethylene is typically available for commercial and industrial purposes in a c.P. grade (minimum purity of 99.5 mole percent), and a technical grade (minimum purity of 98.1 mole percent).
Properties of Ethylene
| Melting point: | −169 °C(lit.) |
| Boiling point: | −104 °C(lit.) |
| Density | 0.00126 |
| vapor density | 0.97 (vs air) |
| vapor pressure | 35.04 atm ( 20 °C) |
| refractive index | 1.363 |
| Flash point: | -100 °C |
| form | Colorless gas |
| explosive limit | 36% |
| Water Solubility | 90.91g/L(25 ºC) |
| FreezingPoint | -169.4℃ |
| Merck | 13,3825 |
| BRN | 1730731 |
| Stability: | Stable. Highly flammable - note wide explosion limits. Incompatible with strong oxidizing agents. Readily forms explosive mixtures with air. |
| CAS DataBase Reference | 74-85-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Ethylene(74-85-1) |
| IARC | 3 (Vol. Sup 7, 60) 1994 |
| EPA Substance Registry System | Ethylene (74-85-1) |
Safety information for Ethylene
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Gas Cylinder Compressed Gases GHS04  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H220:Flammable gases H280:Gases under pressure H336:Specific target organ toxicity,single exposure; Narcotic effects |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P405:Store locked up. P403+P233:Store in a well-ventilated place. Keep container tightly closed. P410+P403:Protect from sunlight. Store in a well-ventilated place. |
Computed Descriptors for Ethylene
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 74-85-1 Ethylene 98%View Details
74-85-1 Ethylene 98%View Details
74-85-1 -
 74-85-1 99%View Details
74-85-1 99%View Details
74-85-1 -
 Ethylene 98%View Details
Ethylene 98%View Details
74-85-1 -
 Ethylene 74-85-1 98%View Details
Ethylene 74-85-1 98%View Details
74-85-1 -
 Ethylene CAS 74-85-1View Details
Ethylene CAS 74-85-1View Details
74-85-1 -
 Semiconductor Ethylene Gas Sensor, 200 Gram, 24VView Details
Semiconductor Ethylene Gas Sensor, 200 Gram, 24VView Details
74-85-1 -
 Fixed Ethylene Gas DetectorView Details
Fixed Ethylene Gas DetectorView Details
74-85-1 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0
