Hydrogen Sulfide
- CAS NO.:7783-06-4
- Empirical Formula: H2S
- Molecular Weight: 34.08
- MDL number: MFCD00011444
- EINECS: 231-977-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
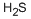
What is Hydrogen Sulfide?
Description
Hydrogen Sulfide is a colorless, very poisonous, flammable gas with the
characteristic foul odor of rotten eggs. It often results
from the bacterial breakdown of organic matter in the
absence of oxygen, such as in swamps and sewers where
anaerobic digestion can take place. It also occurs in
volcanic gases, “natural gas”, and some well waters.
Hydrogen sulfide has numerous names, some of which
are archaic.
Small amounts of hydrogen sulfide occur in crude oil,
but natural gas can contain up to 90%. About 10% of the
total global emission of H2S is due to human activity.
Description
Hydrogen sulfide (H2S) is a dense, colorless gas. At low concentrations, it has a characteristic “rotten egg” odor; at high concentrations, it is extremely toxic and even explosive. It is abundant in nature, mainly as a result of the anaerobic decay of sulfur-containing organic matter. It is also produced in large quantities in oil refineries from the desulfurization of petroleum fractions.
Credit for the discovery of H2S is usually attributed to Swedish chemist C. W. Scheele in 1777. Because the byproduct H2S is made in large quantities, there is little need to manufacture it “on purpose”. Almost all refinery H2S is reduced to nontoxic elemental sulfur via the venerable Claus process, invented by German chemist. C. F. Claus in 1883.
H2S has the formula of an inorganic acid; but in aqueous solution, the dissociation constant (Ka) for the first proton is only ≈1 x 10–7. The second proton’s?Ka?is a miniscule 1 x 10–12. H2S precipitates many inorganic cations [e.g., Cu(II), Pb(II), and Hg(II)], making it useful for the qualitative detection of these ions.
Chemical properties
Hydrogen sulfide is a colourless gas with strong odour of rotten eggs (odour threshold ca 0.2 ppt).
Physical properties
H2S is soluble in carbon disulfide, methanol, acetone and alkanolamines. A solution of hydrogen sulfide in water is initially clear but over time turns cloudy. This is due to the slow reaction of hydrogen sulfide with the oxygen dissolved in water, yielding elemental sulfur, which precipitates out.
Occurrence
Reported found in heated French beans, beef broth, vapors of canned beef, canned beef, beef extract, heated beef fat, raw beef, beer, bread, heated Brussels sprouts, cabbage, cooked celery, cheddar cheese, cooked and raw chicken, chives, heated coconut, codfish, ground and roast coffee, heated corn, heated egg, grapefruit juice, cooked herring, citrus juices, strawberry, cabbage, onion, potato, rutabaga, tomato, blue cheese, buttermilk, raw and boiled eggs, coffee, potato chips, rice, soybeans, okra, sweet corn, sake, squid, shrimps, cooked, fatty fish and other natural sources
History
Hydrogen sulfide is a colorless, flammable, toxic gas with the characteristic odor of rotten eggs.It is produced naturally from the anaerobic bacterial decomposition of organic wastes, occurs in volcanic gases and hot springs, is a product of animal digestion, and is generated in industrial processes. Hydrogen is a natural component of natural gas and petroleum; it is only a small fraction of oil (hundreds of ppm), but may form an appreciable component of natural gas. Natural gas typically contains up to 5% hydrogen sulfide. Natural gas is considered sour if the hydrogen sulfide content exceeds 5.7 mg of H2S per cubic meter of natural gas. The process for removing hydrogen sulfide from sour gas is referred to as sweetening the gas. Because hydrogen sulfide is associated with anaerobic respiration in sewers and swamps, it is referred to as sewer gas, swamp gas, or stink damp.
The Uses of Hydrogen Sulfide
Hydrogen sulfide is used as an analyticalreagent and in the manufacture of heavywater. It occurs in natural gas and sewer gas.It is formed by the reaction of a metal sulfidewith dilute mineral acid, and in petroleumrefining.
The Uses of Hydrogen Sulfide
To produce elemental sulfur and sulfuric acid; in manufacture of heavy water and other chemicals; in metallurgy; as analytical reagent.
The Uses of Hydrogen Sulfide
Hydrogen sulfide has relatively few commercial uses. It is used to produce elementalsulfur, sulfuric acid, and heavy water for nuclear reactors.
Definition
ChEBI: A sulfur hydride consisting of s single sulfur atom bonded to two hydrogen atoms. A highly poisonous, flammable gas with a characteristic odour of rotten eggs, it is often produced by bacterial decomposition of organic matter in the absence of oxygen.
Production Methods
Hydrogen sulfide is produced during anaerobic respiration (fermentation). Anaerobic respirationenables organisms, primarily bacteria and other microbes, to meet their energy needsusing sulfate, elemental sulfur, and sulfur compounds as electron acceptors instead of oxygen.
Preparation
By far the largest industrial route toH2S occurs in petroleum refineries. The “hydrodesulfurization” process liberates sulfur from petroleum by the action of hydrogen. The resulting H2S is converted to elemental sulfur by partial combustion via the Claus process that is a major source of elemental sulfur (In the Claus process, hydrogen sulfide is catalytically reacted with oxygen from the air to produce sulfur and sulfur dioxide). Other anthropogenic sources of hydrogen sulfide include coke ovens, paper-mills (using the sulfate method), and tanneries, where Na2S is used for processing cowhide to form leather. H2S arises from virtually anywhere where elemental sulfur comes in contact with organic material, especially at high temperatures.
Reactions
Fluorine, chlorine, bromine, and iodine react with H2S to form the corresponding halogen acid. Metal sulfides are formed when H2S is passed into solutions of the heavy metals, such as Ag, Pb, Cu, and Mn. This reaction is responsible for the tarnishing of Ag and is the basis for the separation of these metals in classical wet qualitative analytical methods. Hydrogen sulfide reacts with many organic compounds.
Aroma threshold values
Detection: 10 ppb
Air & Water Reactions
Highly flammable; a flame can very easily flash back to the source of leak. Soluble in water to a maximum of 0.4% by mass at room temperature .
Reactivity Profile
HYDROGEN SULFIDE reacts as an acid and as a reducing agent. Explodes on contact with oxygen difluoride, bromine pentafluoride, chlorine trifluoride, dichlorine oxide, silver fulminate. May ignite and explode when exposed to powdered copper in oxygen [Mertz, V. et al., Ber., 1880, 13, p. 722]. May react similarly with other powdered metals. Ignites on contact with metal oxides and peroxides (barium peroxide, chromium trioxide, copper oxide, lead dioxide, manganese dioxide, nickel oxide, silver oxide, silver dioxide, thallium trioxide, sodium peroxide, mercury oxide, calcium oxide) [Mellor, 1947, vol. 10, p. 129, 141]. Ignites with silver bromate, lead(II) hypochlorite, copper chromate, nitric acid, lead(IV) oxide and rust. May ignite if passed through rusty iron pipes [Mee, A. J., School Sci. Rev., 1940, 22(85), p. 95]. Reacts exothermically with bases. The heat of the reaction with soda lime, sodium hydroxide, potassium hydroxide, barium hydroxide may lead to ignition or explosion of the unreacted portion in the presence of air / oxygen [Mellor, 1947, vol. 10, p. 140].
Hazard
Highly flammable, dangerous fire risk, explosive limits in air 4.3–46%. Toxic by inhalation, strong irritant to eyes and mucous membranes.
Health Hazard
The acute toxicity of hydrogen sulfide by inhalation is moderate. A 5-min exposure to 800 ppm has resulted in death. Inhalation of 1000 to 2000 ppm may cause coma after a single breath. Exposure to lower concentrations may cause headache, dizziness, and upset stomach. Low concentrations of H2S (20 to 150 ppm) can cause eye irritation, which may be delayed in onset. Although the odor of hydrogen sulfide is detectable at very low concentrations, it rapidly causes olfactory fatigue at higher levels, and therefore is not considered to have adequate warning properties. Hydrogen sulfide has not been shown to be carcinogenic or to have reproductive or developmental effects in humans
Health Hazard
Hydrogen sulfide is a highly toxic gas. Exposure to high concentrations can result inunconsciousness and respiratory paralysis.A 5-minute exposure to a concentration of1000 ppm can be lethal to humans. Prolonged exposure to concentrations between250 and 500 ppm can cause respiratory irri tation, congestion of the lung, and bronchialpneumonia. Toxic symptoms that have beennoted from occupational exposure to hydrogen sulfide in a heavy water plant areheadache, nausea, cough, nervousness, andinsomnia (ACGIH 1986). In addition, it isan irritant to the eyes. Conjunctivities mayresult from exposure to 20–30 ppm.
Fire Hazard
Compound is heavier than air and may travel a considerable distance to source of ignition and flash back. HYDROGEN SULFIDE forms explosive mixtures with air over a wide range. Also reacts explosively with bromine pentafluoride, chlorine trifluoride, nitrogen triiodide, nitrogen trichloride, oxygen difluoride, and phenyl diazonium chloride. When heated to decomposition, HYDROGEN SULFIDE emits highly toxic fumes of oxides of sulfur. Incompatible with many materials including strong oxidizers, metals, strong nitric acid, bromine pentafluoride, chlorine trifluoride, nitrogen triiodide, nitrogen trichloride, oxygen difluoride and phenyl diazonium chloride. Avoid physical damage to containers; sources of ignition; storage near nitric acid, strong oxidizing materials, and corrosive liquids or gases.
Flammability and Explosibility
Hydrogen sulfide is flammable in air in the range of 4.3 to 45.5% (NFPA rating = 4). Combustion products (sulfur oxides) are also toxic by inhalation. In the event of a hydrogen sulfide fire, stop the flow of gas if possible without risk of harmful exposure and let the fire burn itself out.
Agricultural Uses
Hydrogen sulphide (H2S) is a colorless, poisonous, flammable gas with an odor of rotting eggs. It is found in cesspools and mines and is a by-product of decomposed substances containing sulphur. It is one of the gaseous end-products of the reduction of sulphate in highly degraded paddy fields. Hydrogen sulphide is also produced in the laboratory for use as an analytical reagent.
Materials Uses
Dry hydrogen sulfide is satisfactorily handled under pressure at normal ambient temperatures in carbon steel or black iron piping. Carbon steels in wet applications are known to be subject to sulfide stress cracking and low- temperature brittle fracture under some conditions of temperature, stress, and pressure. While hydrogen sulfide itself is relatively noncorrosive to steel in many uses, factors such as impurities, pH, erosive conditions, and high thermal or mechanical stresses in the metal can cause severe corrosion problems. High-strength steels are subject to crack formation when exposed to hydrogen sulfide.
Synthesis
Hydrogen sulfide gas can be formed and released whenever waste containing sulfur is broken down by bacteria.
Potential Exposure
ReproductiveEffector; Human Data. Hydrogen sulfide is used in the syn-thesis of inorganic sulfides, sulfuric acid, and organic sulfurcompounds; as an analytical reagent; as a disinfectant inagriculture; and in metallurgy. It is generated in many industrial processes as a by-product and also during thedecomposition of sulfur-containing organicmatter,sopotential for exposure exists in a variety of situations.Hydrogensulfide is found in natural gas, volcanic gas, andin certain natural spring waters. Its may also be encounteredin the manufacture of barium carbonate, barium salt, cello-phane,depilatories, dyes, pigments, felt, fertilizer, adhe-sives, viscoserayon, lithopone, synthetic petroleumproducts; in the processing of sugar beets; in mining, partic-ularly where sulfide ores are present; in sewers and sewagetreatment plants; during excavation of swampy or filledground for tunnels, wells, and caissons; during drilling ofoil and gas wells; in purification of hydrochloric acid andphosphates; during the low-temperature carbonization ofcoal; in tanneries, breweries, slaughterhouses; in fat render-ing; and in lithography and photoengraving.
Physiological effects
Hydrogen sulfide is a toxic, irritating, and asphyxiant
gas. The substance is known to be produced and metabolized naturally in the human
body at low concentrations, but can be
quickly fatal once the natural bodily defenses
are overwhelmed.
ACGIH recommends a Threshold Limit
Value-Time-Weighted Average (TLV-TWA)
of 10 ppm (14 mg/m3
) for hydrogen sulfide. The
TLV- TWA is the time-weighted average concentration
for a normal 8-hour workday and a
40-hour workweek, to which nearly all workers
may be repeatedly exposed, day after day, without
adverse effect.
ACGIH recommends a Threshold Limit
Value-Short Term Exposure Limit (TLVSTEL)
of 15 ppm (21 mg/m3
) for hydrogen sulfide.
The TLV-STEL is the IS-minute TWA
exposure that should not be exceeded at any
time during a workday even if the 8-hour TWA
is within the TLV- TWA. Exposures above the
TLV- TWA up to the STEL should not be
longer than 15 minutes and should not occur
more than 4 times per day. There should be at
least 60 minutes between successive exposures
in this range.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek med-ical attention immediately. If frostbite has occurred, seekmedical attention immediately; do NOT rub the affectedareas or flush them with water. In order to prevent furthertissue damage, do NOT attempt to remove frozen clothingfrom frostbitten areas. If frostbite has NOT occurred, imme-diately and thoroughly wash contaminated skin with soapand water. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precau-tions, including resuscitation mask) if breathing has stoppedand CPR if heart action has stopped. Transfer promptly to amedical facility. Medical observation is recommended for24- -48 h after breathing overexposure, as pulmonary edemamay be delayed. As first aid for pulmonary edema, a doctoror authorized paramedic may consider administering a corti-costeroid spray.
Environmental Fate
Hydrogen sulfide is a colorless, flammable compressed liquid gas with a characteristic odor of rotten eggs. The solubility of hydrogen sulfide in water is 3980 mg l-1 at 20 ℃ and it is soluble in certain polar organic solvents, notably methanol, acetone, propylene carbonate, sulfolane, tributyl phosphate, various glycols and glycol ethers, gasoline, kerosene, crude oil, and carbon disulfide. The calculated vapor pressure at 21.9 ℃ is 1929 Pa. Boiling point and melting point of the substance are -60.33 ℃ and-85.49 ℃, respectively. Based on the estimated Henry’s law constant of 468 atm mol-1 for hydrogen sulfide, volatilization from water and soil is high.
storage
cylinders of hydrogen sulfide should be stored and used in a continuously ventilated gas cabinet or fume hood. Local fire codes should be reviewed for limitations on quantity and storage requirements.
Shipping
Hydrogen sulfide requires a shipping label of“POISON GAS, FLAMMABLE GAS.”It falls in HazardClass 2.3. It is a violation of transportation regulations torefill compressed gas cylinders without the express writtenpermission of the owner.
Purification Methods
Wash it, then pass the gas through a train of tubes containing saturated Ba(OH)2 (2x), water (2x), and dilute HCl [Goates et al. J Am Chem Soc 73 707 1951]. It is available in gas cylinders. HIGHLY POISONOUS.
Toxicity evaluation
Toxicity of hydrogen sulfide is most likely related to inhibition of metal-containing enzymes such as cytochrome oxidase, the final enzyme of the mitochondrial respiratory chain, and carbonic anhydrase. Therefore, hydrogen sulfide affects cellular energy production and respiration. Mucous membranes and tissues with a high oxygen demand, like nervous and cardiac tissues are most susceptible tissues in exposure to hydrogen sulfide. In addition, sulfide also seems to act on the respiratory drive through other mechanisms such as suppression of synaptic activity, inhibition of monoamine oxidase, a direct action on respiratory centers in the brain, and stimulation of the glutamate receptors in the brain. The hydrosulfide anion also forms a complex with methemoglobin and creates sulfmethemoglobin. On the other hand, hydrosulfide can be produced endogenously, particularly in mammalian cells, through an enzymatic pathway and in a smaller part via a nonenzymatic pathway. Among enzymes involved in hydrosulfide production, cystathionine- synthase and cystathionine-lyase have been investigated extensively; both use vitamin B6 as a cofactor. Captopyruvate sulfurtransferase along with cysteine aminotransferase are involved in transsulfuration and reverse transsulfuration pathways in different capacities and utilize specific substrates. Of course, the regulation mechanisms for the expression and activities of these hydrosulfide-generating enzymes under physiological or pathophysiological conditions needs more research. These enzymes are differentially expressed in neuronal, immune, cardiovascular, renal, gastrointestinal, reproductive, respiratory, liver, and endocrine systems and affect the functions of these systems through production of hydrosulfide. Meanwhile, different molecular targets, such as different ion channels and signaling proteins, mediate physiological functions of hydrogen sulfide. Alternations of hydrosulfide metabolism lead to an array of pathological disturbances in the form of hypertension, diabetes, cirrhosis, atherosclerosis, heart failure, inflammation, sepsis, erectile dysfunction, asthma, and neurodegenerative disease.
Incompatibilities
Hydrogen sulfide is incompatible with strong oxidizers. It will attack many metals, forming sulfides. Liquid hydrogen sulfide will attack some forms of plastics, rubber, and coatings. H2S reacts violently with a variety of metal oxides, including the oxides of chromium, mercury, silver, lead, nickel, and iron.
Waste Disposal
To respond to a release, use appropriate protective equipment and clothing. Positive pressure air-supplied respiratory protection is required. Close cylinder valve and ventilate area. Remove cylinder to a fume hood or remote area if it cannot be shut off. Disposal Excess hydrogen sulfide should be returned to the manufacturer, according to your institution's waste disposal guidelines. For more information on disposal procedures, see Chapter 7 of this volume.
GRADES AVAILABLE
Hydrogen sulfide is available in a technical or commercial grade that is 99 mole percent minimum hydrogen sulfide. It is also available in higher purities, up to 99.99 mole percent minimum hydrogen sulfide.
Properties of Hydrogen Sulfide
| Melting point: | −85 °C(lit.) |
| Boiling point: | −60 °C(lit.) |
| Density | dgas 1.19 (air = 1.00) |
| vapor density | 1.19 (15 °C, vs air) |
| vapor pressure | 252 psi ( 21 °C) |
| FEMA | 3779 | HYDROGEN SULFIDE |
| Flash point: | -17℃ |
| storage temp. | 2-8°C |
| solubility | soluble in H2O |
| form | colorless gas |
| pka | 7(at 25℃) |
| color | colorless gas; flammable |
| Odor | Strong rotten egg odor detectable at 0.001 to 0.1 ppm (mean = 0.0094 ppm);
olfactory fatigue occurs quickly at high concentrations |
| explosive limit | 6% |
| Odor Threshold | 0.00041ppm |
| Water Solubility | 1g dissolves in H2O: 187mL (10°C), 242mL (20°C), 314 (30°C) [MER06] |
| Merck | 13,4823 |
| BRN | 3535004 |
| Dielectric constant | 9.3(-84℃) |
| Stability: | Stable. Highly flammable. May form explosive mixture with air. Note wide explosive limits. Incompatible with strong oxidizing agents, many metals. May react violently with metal oxides, copper, fluorine, sodium, ethanal. |
| CAS DataBase Reference | 7783-06-4(CAS DataBase Reference) |
| EPA Substance Registry System | Hydrogen sulfide (7783-06-4) |
Safety information for Hydrogen Sulfide
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Gas Cylinder Compressed Gases GHS04  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H220:Flammable gases H280:Gases under pressure H330:Acute toxicity,inhalation H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P273:Avoid release to the environment. P284:Wear respiratory protection. P410+P403:Protect from sunlight. Store in a well-ventilated place. |
Computed Descriptors for Hydrogen Sulfide
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran
You may like
-
 7783-06-4 Hydrogen sulfide 98%View Details
7783-06-4 Hydrogen sulfide 98%View Details
7783-06-4 -
 7783-06-4 98%View Details
7783-06-4 98%View Details
7783-06-4 -
 17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 -
 131987-69-4 98+View Details
131987-69-4 98+View Details
131987-69-4 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
5968-21-8 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
