Sulfathiazole sodium
Synonym(s):4-Amino-N-(2-thiazolyl)benzenesulfonamide sodium salt
- CAS NO.:144-74-1
- Empirical Formula: C9H10N3NaO2S2
- Molecular Weight: 279.31
- MDL number: MFCD00072133
- EINECS: 205-638-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-06 18:33:45
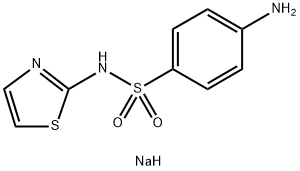
What is Sulfathiazole sodium?
Description
Sulfathiazole sodium salt is a short-acting sulfa drug. It was a common oral and topical antibiotic until less toxic alternatives were discovered. It is no longer used in humans. Sulfathiazole is added to the diet of laboratory animals to inhibit folate formation by gut bacteria. This ensures that the animal′s only source of available folate is from their diet. Sulfathiazole is a sulfonamide antibiotic that blocks the synthesis of dihydrofolic acid by inhibiting the enzyme dihydropteroate synthase. Sulfathiazole is a competitive inhibitor of bacterial para-aminobenzoic acid (PABA), which is required for bacterial synthesis of folic acid. It is active against Gram positive bacteria, Gram negative bacteria and Chlamydia. Mode of resistance is via the alteration of dihydropteroate synthase or alternative pathway for folic acid synthesis.
The Uses of Sulfathiazole sodium
Sulfathiazole sodium salt is a competitive inhibitor of bacterial para-aminobenzoic acid (PABA). It can also be used in biological study of thermotherapy and sulfonamide antibiotics deciphered Cyanobacteria and Proteobacteria microbiome in huanglongbing-affected Citrus paradise and Citrus limon.
What are the applications of Application
Sulfathiazole sodium salt is a competitive inhibitor of bacterial para-aminobenzoic acid (PABA)
General Description
Chemical structure: sulfonamide
Biochem/physiol Actions
Sulfathiazole is a sulfonamide antibiotic that blocks the synthesis of dihydrofolic acid by inhibiting the enzyme dihydropteroate synthase. Sulfathiazole is a competitive inhibitor of bacterial para-aminobenzoic acid (PABA), which is required for bacterial synthesis of folic acid. It is active against Gram positive bacteria, Gram negative bacteria and Chlamydia. Mode of resistance is via the alteration of dihydropteroate synthase or alternative pathway for folic acid synthesis.
Properties of Sulfathiazole sodium
| storage temp. | Store at -20°C |
| solubility | H2O: soluble50mg/mL |
| form | powder |
| color | white to off-white |
| BRN | 3802297 |
| Stability: | Hygroscopic |
| InChI | InChI=1S/C9H9N3O2S2.Na.H/c10-7-1-3-8(4-2-7)16(13,14)12-9-11-5-6-15-9;;/h1-6H,10H2,(H,11,12);; |
| CAS DataBase Reference | 144-74-1(CAS DataBase Reference) |
| EPA Substance Registry System | Benzenesulfonamide, 4-amino-N-2-thiazolyl-, monosodium salt (144-74-1) |
Safety information for Sulfathiazole sodium
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Sulfathiazole sodium
| InChIKey | PLYRQFRFHFKJLU-UHFFFAOYSA-N |
| SMILES | S(C1C=CC(N)=CC=1)(=O)(=O)NC1SC=CN=1.[NaH] |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE 4-Bromopyrazole 5,6-Dimethoxyindanone Tert-butyl bis(2-chloroethyl)carbamate (2-Hydroxyphenyl)acetonitrile 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Sulfathiazole sodium salt 95.00% CAS 144-74-1View Details
Sulfathiazole sodium salt 95.00% CAS 144-74-1View Details
144-74-1 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1