Sulbactam
Synonym(s):(2S,5R)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid 4,4-dioxide;Penicillanic acid 1,1-dioxide;Penicillanic acid dioxide;Penicillanic acid S,S-dioxide;Penicillanic acid sulfone
- CAS NO.:68373-14-8
- Empirical Formula: C8H11NO5S
- Molecular Weight: 233.24
- MDL number: MFCD00867005
- EINECS: 269-878-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 11:34:44
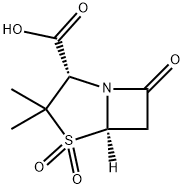
What is Sulbactam?
Absorption
Peak serum concentrations are reached almost immediately following a 15-minute intravenous infusion of sulbactam + ampicillin. Mean peak serum levels for sulbactam range from 48 to 88 mcg/mL following intravenous administration of 2000 mg of ampicillin plus 1000 mg sulbactam. After an intramuscular injection of 1000 mg ampicillin plus 500 mg sulbactam, peak sulbactam serum levels ranging from 6 to 24 mcg/mL are attained.
Description
Sulbactam is prepared by partial chemical synthesis from penicillins. The oxidation of the sulfur atom to a sulfone greatly enhances the potency of sulbactam. The combination of sulbactam and ampicillin (Unasyn) is now clinically popular.
Originator
Sulbactam-Sodium,Antibiotic Co.,Bulgaria
The Uses of Sulbactam
Sulbactam sodium is a semi-synthetic penem antibiotic formed by the oxidation of penicillanic acid to its sulfone and was invented by Barth at Pfizer in 1978. Sulbactam sodium is a weak antibiotic but its action as an irreversible inhibitor of β-lactamase is exploited to block the degradation of other penicillin derivatives. Sulbactam acts as a synergist with cephalosporins and penicillins against Gram positive bacteria and is used commercially in combination with ampicillin.
The Uses of Sulbactam
antidepressant, dopamine uptake inhibitor
The Uses of Sulbactam
A β-lactamase inhibitor.
What are the applications of Application
Sulbactam is a β-lactamase inhibitor
Indications
Sulbactam is currently available in combination products with ampicillin. Within this formulation it is indicated for the treatment of infections due to susceptible strains of the designated microorganisms in the conditions listed below. Skin and Skin Structure Infections caused by beta-lactamase producing strains of Staphylococcus aureus, Escherichia coli, Klebsiella spp. (including K. pneumoniae), Proteus mirabilis, Bacteroides fragilis, Enterobacter spp., and Acinetobacter calcoaceticus. Intra-Abdominal Infections caused by beta-lactamase producing strains of Escherichia coli, Klebsiella spp. (including K. pneumoniae), Bacteroides spp. (including B. fragilis), and Enterobacter spp. Gynecological Infections caused by beta-lactamase producing strains of Escherichia coli, and Bacteroides spp. (including B. fragilis).
Background
Sulbactam is a β-lactamase inhibitor given in combination with β-lactam antibiotics to inhibit β-lactamase, an enzyme produced by bacteria that destroys antibiotic activity.
Definition
ChEBI: Sulbactam is a member of penicillanic acids.
Manufacturing Process
Sulbactam sodium is semi-synthetic antibiotic of penicillinic group. Start
material for it's synthesis is 6-aminopenicillanic acid. First 6-aminopenicillanic
acid was isolated in 1957 year from benzylpenicilline as resalt of treating of it
by penicillinaze. Benzylpenicilline is produced by microorganism of genus
Streptomyces.
Further, 6-aminopenicillanic acid reacted with bromine, hydrochloric acid and
NaNO2. As a result the 6,6-dibromopenicillanic acid was obtained.
6,6-Dibromopenicillanic acid was oxidized by KMnO4, to give 6,6-dibromo-1,1-The 6,6-dibromo-1,1-dioxopenicillanic acid in presence of Fe was converted to
the 1,1-dioxopenicillanic acid (sulbactam acid). The sulbactam acid was
treated by sodium 2-ethylhexanoate and crude sulbactam sodium was
obtained.
Therapeutic Function
Beta-lactamase inhibitor
Antimicrobial activity
Sulbactam has very weak antimicrobial activity against most bacteria. Its only notable activity is against N. gonorrhoeae, N. meningitidis and Acinetobacter baumannii.
Clinical Use
Sulbactam is penicillanic acid sulfone or 1,1-dioxopenicillanicacid. This synthetic penicillin derivative is a potent inhibitorof S. aureus β-lactamase as well as many β-lactamaseselaborated by Gram-negative bacilli. Sulbactam has weak intrinsicantibacterial activity but potentiates the activity ofampicillin and carbenicillin against β-lactamase–producingS. aureus and members of the Enterobacteriaceae family. Itdoes not, however, synergize with either carbenicillin or ticarcillinagainst P. aeruginosa strains resistant to these agents.Failure of sulbactam to penetrate the cell envelope is a possibleexplanation for the lack of synergy.
Fixed-dose combinations of ampicillin sodium and sulbactamsodium, marketed under the trade name Unasyn assterile powders for injection, have been approved for use inthe United States. These combinations are recommended forthe treatment of skin, tissue, intra-abdominal, and gynecologicalinfections caused by β-lactamase–producing strainsof S. aureus, E. coli, Klebsiella spp., P. mirabilis, B. fragilis,and Enterobacter and Acinetobacter spp.
Metabolism
Not Available
Properties of Sulbactam
| Melting point: | 154-157℃ |
| Boiling point: | 567.7±50.0 °C(Predicted) |
| alpha | D20 +251° (c = 0.01 in pH 5.0 buffer) |
| Density | 1.62±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | H2O: ≥18mg/mL |
| form | lyophilized powder |
| pka | 2.62±0.40(Predicted) |
| color | white to tan |
| optical activity | [α]/D ≥+225°, c = 1 in H2O |
| Water Solubility | Soluble in water at 18mg/ml |
| Merck | 14,8889 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 68373-14-8 |
Safety information for Sulbactam
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for Sulbactam
| InChIKey | FKENQMMABCRJMK-RITPCOANSA-N |
Sulbactam manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Sulbactam 98% CAS 68373-14-8View Details
Sulbactam 98% CAS 68373-14-8View Details
68373-14-8 -
 Sulbactam CAS 68373-14-8View Details
Sulbactam CAS 68373-14-8View Details
68373-14-8 -
 Sulbactam CAS 68373-14-8View Details
Sulbactam CAS 68373-14-8View Details
68373-14-8 -
 Sulbactam CAS 68373-14-8View Details
Sulbactam CAS 68373-14-8View Details
68373-14-8 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
