Strontium sulfide
- CAS NO.:1314-96-1
- Empirical Formula: SSr
- Molecular Weight: 119.68
- MDL number: MFCD00049560
- EINECS: 215-249-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16
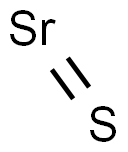
What is Strontium sulfide?
Chemical properties
Strontium sulfide is a grey powder giving a hydrogen sulphide-like smell in the air. It is insoluble in cold water, and decomposes in hot water and acid. In the wet air can decompose to H2S, can also be oxidised to SrSO4. heating for 3 hours to light red, and water vapour contact with the formation of SrO, SrSO4, H2 and SO2. reaction with acid to produce H2S.
The Uses of Strontium sulfide
Strontium sulfide (SrS) smells like rotten eggs. It is used as a depilatory to remove hair from skin and hides.
Production Methods
Strontium sulfide, grayish-white solid (thermodynamic Ksp 500) reactive with water to form strontium hydrosulfide, Sr(SH)2, solution. Strontium hydrosulfide is formed (1) by reaction of strontium sulfide and H2O, (2) by saturation of strontium hydroxide solution with H2S. Strontium polysulfides are formed by boiling strontium hydrosulfide with sulfur.
Preparation
Strontium sulfide can be
prepared by the direct reaction of the elements, calcined
in an inert atmosphere, at a 1:1.05 molecular ratio:
Sr+S+heat→SrS
SrS can also be produced by the “carbothermic reduction”
of calcium sulfate, which entails the conversion of
carbon, usually as charcoal, to CO2:
SrSO4+2C→SrS+ 2CO2
This can react further:
3SrSO4+SrS→4SrO+ 4SO2
A mixture of oxide-sulfide is the result. Thus, this
type of preparation cannot be used to prepare a pure
SrS salt.
General Description
Gray powder with an odor of H2S in moist air.
Air & Water Reactions
Slowly releases H2S in moist air.
Reactivity Profile
STRONTIUM SULFIDE reacts vigorously with acids to release hydrogen sulfide gas. May react exothermically with oxidizing agents including inorganic oxoacids, organic peroxides and epoxides, and inorganic peroxides to generate toxic gases.
Hazard
Moderate fire and explosion risk. Irritant to skin and tissues.
Health Hazard
ACUTE/CHRONIC HAZARDS: Irritant to skin and tissue. Moderate fire hazard and explosion risk.
Flammability and Explosibility
Non flammable
Safety Profile
Poison by inhalation and ingestion. Ready decomposes to yield H2S. Incompatible with lead(1V) oxide. When heated to decomposition it emits toxic fumes of SOx. See also SULFIDES and STRONTIUM COMPOUNDS.
Properties of Strontium sulfide
| Melting point: | >2000 °C(lit.) |
| Density | 3,7 g/cm3 |
| solubility | slightly soluble in H2O; soluble in acid solutions |
| form | Powder |
| color | Gray |
| Specific Gravity | 3.7 |
| Water Solubility | Slightly soluble in water. Decomposes in acids |
| Sensitive | Hygroscopic |
| Merck | 14,8849 |
| Stability: | Stable. Incompatible with acids. |
| CAS DataBase Reference | 1314-96-1(CAS DataBase Reference) |
| EPA Substance Registry System | Strontium sulfide (1314-96-1) |
Safety information for Strontium sulfide
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P310:Immediately call a POISON CENTER or doctor/physician. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Strontium sulfide
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
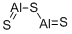
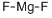
You may like
-
 Strontium sulfide CAS 1314-96-1View Details
Strontium sulfide CAS 1314-96-1View Details
1314-96-1 -
 Strontium sulphide, GR CAS 1314-96-1View Details
Strontium sulphide, GR CAS 1314-96-1View Details
1314-96-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1