Strontium peroxide
Synonym(s):Strontium dioxide
- CAS NO.:1314-18-7
- Empirical Formula: O2Sr
- Molecular Weight: 119.62
- MDL number: MFCD00084686
- EINECS: 215-224-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-10-17 17:11:57
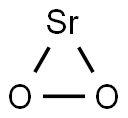
What is Strontium peroxide?
Chemical properties
white to off-white powder
Chemical properties
Strontium peroxide is a lightcolored solid of good thermal stability. Commercial strontium peroxide contains about 85% SrO2 and 10% active oxygen.
The Uses of Strontium peroxide
Strontium Peroxide is an oxidizing agent that is used for bleaching. It has also been used in some pyrotechnic displays to provide a vivid-red color.
The Uses of Strontium peroxide
Strontium peroxide or strontium dioxide (SrO2) can cause fires or explode when heated and in contact with organic substances. It is used as both a reducing agent and an oxidizing agent.
The Uses of Strontium peroxide
The only substantial application for this compound is in pyrotechnics. Strontium peroxide produces a red color in flames.
Preparation
Like the other alkaline earth peroxides, it can be
prepared by reaction of the nitrate and sodium peroxide
in a cold solution:
Sr(NO3)2+ Na2O2+xH2O
SrO2·xH2O+2NaNO3
The hydrated form is usually the octahydrate. If the
anhydrate is desired, the hydrated peroxide is dried
and then sintered at 350°C for 10 min or less:
SrO2·xH2O+ heat→SrO+SrO2+H2O
General Description
A white powder. Insoluble in water and slowly decomposed by water. Noncombustible, but accelerates the burning of combustible material.
Air & Water Reactions
Insoluble in water and slowly decomposed by water.
Reactivity Profile
Strontium peroxide may explode from friction, heat or contamination. Accelerates burning when involved in a fire. May ignite combustibles (wood, paper, oil, clothing, etc.). If the combustible material is finely divided the mixture may be explosive.
Health Hazard
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Fire may produce irritating and/or toxic gases. Toxic fumes or dust may accumulate in confined areas (basement, tanks, hopper/tank cars, etc.). Runoff from fire control or dilution water may cause pollution.
Fire Hazard
May explode from friction, heat or contamination. These substances will accelerate burning when involved in a fire. May ignite combustibles (wood, paper, oil, clothing, etc.). Some will react explosively with hydrocarbons (fuels). Containers may explode when heated. Runoff may create fire or explosion hazard.
Flammability and Explosibility
Not classified
Safety Profile
A powerful oxidizer. A skin, eye, and mucous membrane irritant. Mixtures with organic materials readily ignite with friction or on contact with moisture. See also PEROXIDES and STRONTIUM COMPOUNDS.
Properties of Strontium peroxide
| Melting point: | 215 °C |
| Density | 4.56 g/mL at 25 °C(lit.) |
| solubility | reacts with H2O |
| form | Powder |
| color | Pale brown |
| Water Solubility | Insoluble in water and slowly decomposes in water. |
| Sensitive | Moisture Sensitive |
| Merck | 13,8929 |
| CAS DataBase Reference | 1314-18-7(CAS DataBase Reference) |
| EPA Substance Registry System | Strontium peroxide (Sr(O2)) (1314-18-7) |
Safety information for Strontium peroxide
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for Strontium peroxide
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran



You may like
-
 Strontium peroxide CAS 1314-18-7View Details
Strontium peroxide CAS 1314-18-7View Details
1314-18-7 -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4