PYRITE
Synonym(s):Ferrous disulfide
- CAS NO.:1309-36-0
- Empirical Formula: FeS2
- Molecular Weight: 119.975
- MDL number: MFCD00064690
- EINECS: 215-167-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
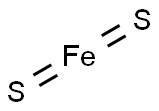
What is PYRITE?
Chemical properties
usually solid chunks
The Uses of PYRITE
Pyrite (FeS2) is more commonly known as fool’s gold. It is used as an iron ore and in the production of sulfur chemicals such as sulfuric acid.
The Uses of PYRITE
For grain mounts, XRD and microprobe standards, identification of unknown minerals.Pyrite is used in the preparation of iron(II) sulfate, sulfur dioxide and iron(II) sulfide. It finds application as semiconductor material, as the cathode material in energizer band and non-rechargeable lithium batteries. It is utilized to make marcasite jewelry. In addition, it is used in photovoltaic solar panels. It is useful for grain mounts, XRD and microprobe standards and identification of unknown minerals.
Definition
ChEBI: An iron sulfide mineral with formula FeS2.
Agricultural Uses
Iron pyrite, also called fool's gold because of its superficial resemblance to gold, is a brassy-yellow or brown tarnished mineral with metallic luster. It is iron disulphide, crystallizing in the cubic system, occurring in octahedral, pyritohedra, etc. Iron pyrite is used in the manufacture of sulphur, sulphuric acid, sulphur dioxide, ferrous sulphate and cheap jewelry.
Agricultural Uses
Pyrite or iron pyrite (FeS2) is a mineral containing iron
and sulphur, and occurs in igneous and metamorphic
rocks. It is also found in sedimentary deposits. Pyrite
resembles gold in appearance and hence it is also known
as fool's gold. It is harder than gold, the hardness being 6
to 6.5 on the Mohs' scale. It is the most common and
widespread of the sulphide minerals and is used as a
source of sulphur for the production of sulphuric acid
(H2SO4). Under oxidizing conditions, pyrite readily
alters to iron sulphates and eventually to limonite,
forming gossans, the surface expression for pyrite-rich
mineral deposits.
Soils with excess pyrite become strongly acidic for
cultivation. When drained and aerated enough for
cultivation, these soils are termed acid sulphate soils or
Katteklei (cat clays) in Dutch.
The role of sulphur in pyrites is multidimensional.
Iron pyrite is a potential source of sulphur for correcting
sulphur-deficiency in crops raised on alkaline, calcareous
soils. Iron pyrite prevents iron chlorosis in plants and
possibly leads to an improvement in the availability of
other nutrients such as P, Fe, Mn and Zn. This is because
pyrite not only contains these micronutrients as
impurities but also enhances the availability of these
native forms by increasing acidity. This role of pyrite is
of special significance in calcareous soils where lime-induced
chlorosis is very common in many crops.
Pyrite can be applied at the rate of 250 kg/ha to
correct lime-induced chlorosis, to improve the malleable
cane percentage and the yield of sugar cane on calcareous
soil.
A symbiotic nitrogen fixation has a special
significance in improving soil fertility. Using graded
doses of pyrite (0 to 400 kg/ha), the number and dry
weight of nodules, as well as grain and straw yield of
chickpea increase significantly.
Properties of PYRITE
| Melting point: | 743 °C |
| Density | 5.02 |
| refractive index | 3.08 |
| form | Grains/Chunks, approximately 1.5-4.8mm (0.06-0.19 in.) |
| Water Solubility | Insoluble in water. |
| Stability: | Stable. May react with acids to generate poisonous hydrogen sulfide. |
| EPA Substance Registry System | Pyrite (1309-36-0) |
Safety information for PYRITE
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for PYRITE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Pyrite CAS 1309-36-0View Details
Pyrite CAS 1309-36-0View Details
1309-36-0 -
 Pyrite CAS 1309-36-0View Details
Pyrite CAS 1309-36-0View Details
1309-36-0 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1