Cupric hydroxide
Synonym(s):Cupric hydroxide
- CAS NO.:20427-59-2
- Empirical Formula: CuH2O2
- Molecular Weight: 97.56
- MDL number: MFCD00010968
- EINECS: 243-815-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-11-07 14:45:54
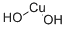
What is Cupric hydroxide?
Chemical properties
Blue powder.Soluble in acids and ammonium hydroxide; insoluble in water.
Chemical properties
Blue, gelatinous or amorphous powder. Insoluble in water.
The Uses of Cupric hydroxide
In manufacture of rayon, battery electrodes, other Cu salts; as mordant in dyeing; as pigment; in fungicides, insecticides; as feed additive; in treating and staining paper; in preparation of Schweitzer's reagent; in catalysts.
The Uses of Cupric hydroxide
Used as a raw material in chemical processing and catalyst manufacturing. Copper(II) hydroxide is used as ceramic colorant. In organic synthesis, it is used as a catalyst to prepare 1-((2-aminoethyl)amino)anthraquinone, 1-amino-4-((2-aminoethyl)amino)anthraquinone by reacting with 1-bromoanthraquinone or 1-amino-4-bromoanthraquinone respectively. It is involved in the synthesis of benzoic acid and octanoic acid. It is used as a precursor in the production of rayon, battery electrodes and other copper salts It is also used as a dye mordant, paper colorant, pigment and feed additive.
What are the applications of Application
Copper(II) hydroxide is a compound also known as Comac parasol
General Description
The orthorhombic nature of copper hydroxide crystals was determined by X ray diffraction. Copper hydroxide can act as a heterogeneous catalyst in the selective oxidative cross coupling of terminal alkynes to yield their corresponding ynamides.
Hazard
Toxic by ingestion and inhalation.
Potential Exposure
Inorganic copper fungicide, nematicide, and microbiocide
Shipping
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Incompatibilities
Reacts with calcium (metal hydroxides), nitroethane, nitromethane, 1-nitropropane, zirconium
Waste Disposal
Copper-containing soluble wastes can be concentrated through the use of ion exchange, reverse osmosis, or evaporators to the point where copper can be electrolytically removed and sent to a reclaiming firm. If recovery is not feasible, the copper can be precipitated through the use of caustics and the sludge deposited in a chemical waste landfill Copper-containing wastes can be concentrated through the use of ion exchange, reverse osmosis, or evaporators to the point where copper can be electrolytically removed and sent to a reclaiming firm. Details of copper recovery from a variety of industrial wastes have been published. If recovery is not feasible, the copper can be precipitated by the use of caustics and the sludge deposited in a chemical waste landfill. Recommendable methods: Precipitation, solidification, landfill, discharge to sewer, & incineration. Peer-review: Precipitate copper with alkali, filter, solidify precipitate. (Do not use ammonia as alkali). Cation exchange will allow recovery of copper. Eluate from cation exchanger can be passed through anion exchanger to remove (or reduce) naphthenic acid content. Exhausted ion exchange resins can be landfilled. (Peer-review conclusions of an IRPTC expert consultation)
Properties of Cupric hydroxide
| Density | 3.4 |
| vapor pressure | 0Pa at 25℃ |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | H2O: insoluble(lit.) |
| form | Powder |
| color | Blue |
| PH | 7.69(1 mM solution);7.69(10 mM solution);7.69(100 mM solution) |
| Water Solubility | Slightly soluble in waterSoluble in acids, ammonium hydroxide, dilute hydrochloric acid, concentrated alkali and potassium cyanide. Insoluble in water, ethanol, and acetone. |
| Sensitive | Hygroscopic |
| Merck | 14,2642 |
| Solubility Product Constant (Ksp) | pKsp: 19.66 |
| Exposure limits | ACGIH: TWA 1 mg/m3 NIOSH: IDLH 100 mg/m3; TWA 1 mg/m3 |
| Stability: | Stable, but hygroscopic. Store in dry conditions. |
| CAS DataBase Reference | 20427-59-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Copper hydroxide(20427-59-2) |
| EPA Substance Registry System | Cupric hydroxide (20427-59-2) |
Safety information for Cupric hydroxide
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H318:Serious eye damage/eye irritation H330:Acute toxicity,inhalation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Cupric hydroxide
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 20427-59-2 Cupric hydroxide 99%View Details
20427-59-2 Cupric hydroxide 99%View Details
20427-59-2 -
 Cupric hydroxide 99%View Details
Cupric hydroxide 99%View Details
20427-59-2 -
 Cupric hydroxide 20427-59-2 99%View Details
Cupric hydroxide 20427-59-2 99%View Details
20427-59-2 -
 Copper(II) hydroxide CAS 20427-59-2View Details
Copper(II) hydroxide CAS 20427-59-2View Details
20427-59-2 -
 Copper(II) hydroxide CAS 20427-59-2View Details
Copper(II) hydroxide CAS 20427-59-2View Details
20427-59-2 -
 Cupric Hydroxide 96% CAS 20427-59-2View Details
Cupric Hydroxide 96% CAS 20427-59-2View Details
20427-59-2 -
 Copper(II) hydroxide CAS 20427-59-2View Details
Copper(II) hydroxide CAS 20427-59-2View Details
20427-59-2 -
 Parikh 50 ppm Copper Hydroxide 50% WP Copper FungicideView Details
Parikh 50 ppm Copper Hydroxide 50% WP Copper FungicideView Details
20427-59-2
