STRONTIUM SELENIDE
- CAS NO.:1315-07-7
- Empirical Formula: HSeSr
- Molecular Weight: 167.6
- MDL number: MFCD00054059
- EINECS: 215-258-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16
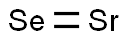
What is STRONTIUM SELENIDE?
Description
Strontium selenide has the molecular formula of SrSe and the molecular weight of 166.5852 g/mol. It is a white cubic crystal whose CAS number is 1315-07-7. Its melting point is 1605°C and its density is 4.54 g/cm3. It reacts with water and so cannot be prepared by an aqueous precipitation method. It can be prepared by the reaction of the oxide with Se metal:
2SrO+ Se+ Heat ? SrSe+ SeO2
It can also be prepared by reducing the selenate with hydrogen gas:
SrSeO4 + 4H2 + heat ? SrSe+ 4H2O
Strontium selenide and barium selenide both have a simple cubic structure, with cube edges equal to 3.117? and 3.308 ?, respectively. In the case of SrSe, the ions at the corners are Sr++ and Se--。
Chemical properties
white cub; -20 mesh with 99.5% purity [CER91] [CRC10]
Physical properties
Phosphors can be categorized in accordance with
their behavior as fluorescent, phosphorescent or stimulable.
As used in this disclosure, such categories
should be understood as based upon the predominant
behavior of the phosphor at about room temperature,
i.e at about 20°C.
? A “stimulable” phosphor is one that, at room
temperature, stores energy absorbed upon exposure
to excitation by electromagnetic radiation and releases
the predominant portion of the stored energy upon
exposure to stimulating electromagnetic radiation.
? A phosphorescent phosphor at room temperature will
store absorbed energy for an appreciable time but will
release the predominant portion of the stored energy
spontaneously.
? A fluorescent phosphor will release the predominant
portion of the absorbed energy as emission radiant
energy substantially simultaneously with exposure to
the exciting radiant energy.
The Uses of STRONTIUM SELENIDE
The major usage of SrSe has been as an infrared stimulable phosphor. Photoluminescent materials useful for detection of infrared light are prepared using a base material, such as SrSe. Up to three dopants, Sm3+, cerium, Ce3+ and Eu2+ compounds are used for the purpose of providing electron traps. The base material can be a combination of alkaline earth metal selenides such as strontium selenide, calcium selenide, or a mixture of these. Luminescence is a long known phenomenon of nature reaching back very far in history. Recorded observations reach back to the last century. Seeback and Becquerel observed momentary visible afterglow in certain materials.
Properties of STRONTIUM SELENIDE
| Melting point: | 1600 °C(lit.) |
| Density | 4.540 |
| form | white cubic crystals |
| color | white cubic crystals, crystalline |
| CAS DataBase Reference | 1315-07-7(CAS DataBase Reference) |
| EPA Substance Registry System | Strontium selenide (SrSe) (1315-07-7) |
Safety information for STRONTIUM SELENIDE
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H331:Acute toxicity,inhalation H373:Specific target organ toxicity, repeated exposure H400:Hazardous to the aquatic environment, acute hazard H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P405:Store locked up. |
Computed Descriptors for STRONTIUM SELENIDE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1