CALCIUM SELENITE
- CAS NO.:856616-83-6
- Empirical Formula: CaO3Se
- Molecular Weight: 167.04
- MDL number: MFCD00049404
- SAFETY DATA SHEET (SDS)

What is CALCIUM SELENITE?
Description
Calcium selenite has the molecular formula of
CaSeO3 and the molecular weight of 167.0410 g/mol. It
can be prepared by the addition of sodium selenite to
a solution of magnesium chloride:
CaCl2(aq)+ Na2SeO3(aq) ?CaSeO3·H2O(solid)
It can also be prepared by treating a solution of
calcium chloride with selenous acid and adding sodium
carbonate to start precipitation. The monohydrate is
a colorless crystal with a density of 2.09 g/cm7.27 that is
insoluble in water. The Ksp is 1.2×10-7.27. It is soluble
in dilute acids. A dihydrate has also been observed
whose molecular weight is 203.0669 g/mol. The monohydrate
has a molecular weight of 185.0569 g/mol and
is a monoclinic crystal with a=7.622?, b =6.745? ,
c=7.922?, b=108.46, V=386.4 ?3, Z=4 with
a density of 3.18 g/cm3. The polyhedron around the
calcium atoms is pentagonal bipyramidal and the selenite
group forms a trigonal pyramid with Se and O
atoms at the apices. These pyramids form layers parallel
to the bc plane which are connected by hydrogen bonds
involving crystal water. The selenite group acts as
a bidentate ligand.
The chemistry of the system CaO–SeO2–H2O has been
investigated at 23 and 80°C. The existence of four major
phases in this system:
CaSeO3·H2O
Ca(HSeO3)·2H2O
Ca2(SeO3)(Se2O5)
Ca2(HSeO3)2
These have been confirmed, and the equilibria among
them determined. At 23°C, CaSeO3·H2O and
Ca(HSeO3)·2H2O are the stable calcium selenites. CaSe-
O3·H2O exists over the pH range 12.5–3.7, making it the
most important phase for natural systems as well as
most technological applications. Ca(HSeO3)·2H2O is
the more acidic phase; it exists over the pH range 3.7–
0.4. Incongruent dissolution of Ca(HSeO3)·2H2O forms
CaSeO3·H2O. These results indicate Ca2(HSeO3)2(Se2O5)
is not a stable phase at 23°C. At 80°C, CaSeO3·H2O,
Ca2(SeO3)(Se2O5), and Ca2(HSeO3)2(Se2O5) are the stable
calcium selenite phases. Ca(HSeO3)·2H2O has not
been observed. Thus, CaSeO3·H2O exists over the
broadest pH range, 11.0–3.9. Ca2(SeO3)(Se2O5) and
Ca2(HSeO3)2(Se2O5) dissolve incongruently.
Properties of CALCIUM SELENITE
| Solubility Product Constant (Ksp) | pKsp: 5.53 |
Safety information for CALCIUM SELENITE
Computed Descriptors for CALCIUM SELENITE
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
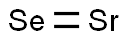

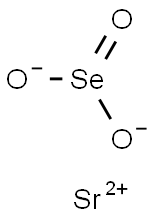
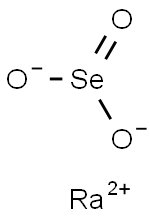
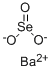
You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4