MAGNESIUM SELENITE
- CAS NO.:15593-61-0
- Empirical Formula: MgO3Se
- Molecular Weight: 151.2632
- MDL number: MFCD00049971
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-10-17 17:11:56
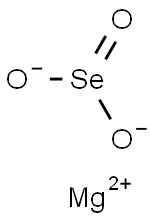
What is MAGNESIUM SELENITE?
Description
Magnesium selenite has the molecular formula of
MgSeO3 and the molecular weight of 151.2658 g/mol.
Its melting point is 557°C. Its CAS number is 15593-
61-0. It is relatively insoluble in water at 0.05454 g/
100 ml. However, the most common form is the hexahydrate
obtained by adding sodium selenite to a solution
of magnesium chloride:
MgCl2(aq)+ Na2SeO3(aq) ?MgSeO3·6H2O(solid)
It can also be prepared by treating a solution of
magnesium chloride with selenous acid and adding
sodium carbonate to start precipitation. The hexahydrate
is a colorless crystal with a density of 2.09 g/cm3
that is insoluble in water. It is soluble in dilute acids. It is orthorhombic in crystal structure and when heated
in a sealed tube to 150°C forms monoclinic prisms of
the dihydrate, when heated to 100°C in air, it loses
5.0 mol of water to form the monohydrate. The monohydrate
then loses the last water of hydration around
190°C to form the anhydrate.
The dihydrate has been the subject of further investigation.
Hydrothermally prepared MgSeO3·2H2O
consists of pairs of edge-sharing MgO6 (4O, 2H2O) octahedra
[dav(Mg–O) = 2.095 ?]. These are linked by pyramidal
SeO3 units [dav(Se–O)=1.697?].
A 7.5 hydrate has also been discovered. The crystal
structure of magnesium selenite as the 7.5-hydrate,
Mg(SeO3)·7.5H2O (space group=P63/mmc), is characterized
by two crystallographically distinct
[Mg(H2O)6]2+
octahedra, one of which is disordered
over two different orientations. The selenite groups
and water molecules (with partially disordered H
atoms) bridge the octahedra via hydrogen bonds.
Chemical properties
colorless ortho-rhomb crystal(s) [LID94] [MER06]
The Uses of MAGNESIUM SELENITE
The selenites, in general, have several versatile and practical applications. The Mg and Ca salts are used as microfertilizers and repellants in agriculture. They are also used as catalysts in organic synthesis and they are precursors for the preparation of pure selenides of stoichiometric proportion. They are also important intermediate products in the production and purification of selenium metal itself.
Properties of MAGNESIUM SELENITE
| Density | 2.090 |
| solubility | insoluble in H2O; soluble in dilute acid solutions |
| form | colorless hexagonal crystals |
| color | colorless hexagonal, hexane crystals, crystalline |
| Water Solubility | insoluble H2O; soluble dilute acids [MER06] |
| Solubility Product Constant (Ksp) | pKsp: 4.89 |
Safety information for MAGNESIUM SELENITE
Computed Descriptors for MAGNESIUM SELENITE
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1