Barium selenite
- CAS NO.:13718-59-7
- Empirical Formula: BaO3Se
- Molecular Weight: 264.29
- MDL number: MFCD00003446
- EINECS: 237-280-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
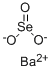
What is Barium selenite?
Description
Barium selenite has the molecular formula of BaSeO3
and the molecular weight of 264.2865 g/mol. Its CAS
number is 13718-59-7. It is a white solid that is insoluble
in water. It can be prepared by the addition of sodium
selenite to a solution of barium chloride:
BaCl2(aq)+ Na2SeO3(aq) ? BaSeO3(solid)
It can also be prepared by treating a solution of
barium chloride with selenous acid and adding sodium
carbonate to start precipitation. There is no record of
a hydrate and the anhydrate is stable upon heating to
over 950 °C.
Chemical properties
solid [ALD93]
Physical properties
The heat of formation of barium selenite is:
△fH298 - BaSeO3 = 2230.6 kcal/mol (—964.7 kJ/mol).
The precipitate of barium selenite is not oxidized by
air during drying at 110°C. In air, however, it oxidizes
to the selenate at about 700–750°C:
2BaSeO3+ O2+ heat ? 2BaSeO4
Bariumselenite is a fine white crystalline powder that
is mainly used in the glass industry to color and decolor
glass.
The Uses of Barium selenite
Barium selenite is primarily chosen for producing special glass and leads to the formation of glass with high index of refraction. Barium selenite has weak oxidizing or reducing powers. Redox reactions can however still occur. The majority of compounds in this class are slightly soluble or insoluble in water. Noncombustible, the substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes of SeO2.
Reactivity Profile
Barium selenite has weak oxidizing or reducing powers. Redox reactions can however still occur. The majority of compounds in this class are slightly soluble or insoluble in water. If soluble in water, then the solutions are usually neither strongly acidic nor strongly basic. These compounds are not water-reactive. Noncombustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes.
Health Hazard
Highly toxic, may be fatal if inhaled, swallowed or absorbed through skin. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Containers may explode when heated. Runoff may pollute waterways.
Flammability and Explosibility
Not classified
Properties of Barium selenite
| solubility | insoluble in H2O |
| form | solid |
| Water Solubility | g/100g solution H2O: 0.005 (0°C), 0.005 (25°C); solid phase, BaSeO3 [KRU93] |
| Exposure limits | ACGIH: TWA 0.5 mg/m3; TWA 0.2 mg/m3 NIOSH: IDLH 50 mg/m3; IDLH 1 mg/m3; TWA 0.5 mg/m3; TWA 0.2 mg/m3 |
| CAS DataBase Reference | 13718-59-7(CAS DataBase Reference) |
| EPA Substance Registry System | Selenious acid, barium salt (1:1) (13718-59-7) |
Safety information for Barium selenite
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H300:Acute toxicity,oral H310:Acute toxicity,dermal H330:Acute toxicity,inhalation H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P320:Specific treatment is urgent (see … on this label). P330:Rinse mouth. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P405:Store locked up. |
Computed Descriptors for Barium selenite
Barium selenite manufacturer
Svaks Biotech India Pvt Ltd
ARRAKIS INDUSTRIES LLP
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


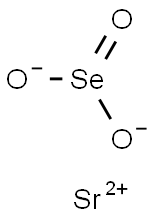
You may like
-
 Barium selenite 98%View Details
Barium selenite 98%View Details
13718-59-7 -
 BARIUM SELENITE 13718-59-7 99%View Details
BARIUM SELENITE 13718-59-7 99%View Details
13718-59-7 -
 13718-59-7 99%View Details
13718-59-7 99%View Details
13718-59-7 -
 BARIUM SULPHATE 13718-59-7 99%View Details
BARIUM SULPHATE 13718-59-7 99%View Details
13718-59-7 -
 Barium Selenite CASView Details
Barium Selenite CASView Details -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1