Tin(IV) chloride
Synonym(s):Tin tetrachloride
- CAS NO.:10026-06-9
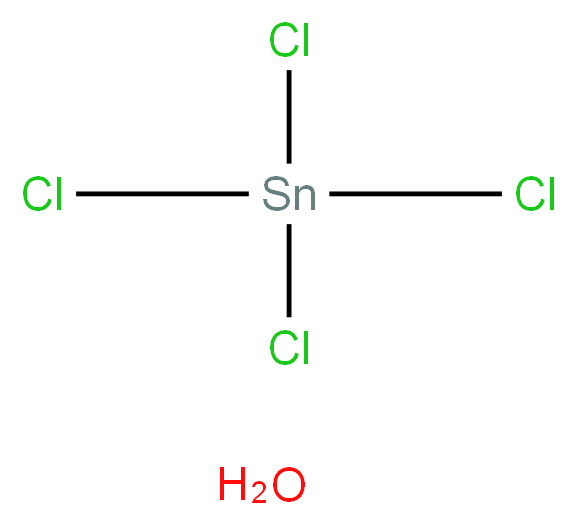
- Empirical Formula: Cl4H2OSn
- Molecular Weight: 278.53
- MDL number: MFCD00149864
- EINECS: 600-048-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
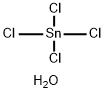
What is Tin(IV) chloride?
Chemical properties
White solid. Soluble in water or alcohol.
Chemical properties
Stannic chloride is a white to yellow powder with a faint odor of HCl.
The Uses of Tin(IV) chloride
Substitute for anhydrous stannic chloride where the presence of water is not objectionable
The Uses of Tin(IV) chloride
Tin (IV) Chloride Pentahydrate is a hydrate of Tin (IV) Chloride(T443805), which can induce slight inhibition of the catalytic activity of horse liver alcohol dehydrogenase (HLADH).
What are the applications of Application
Tin(IV) chloride pentahydrate is a Tin(IV) chloride hydrate
General Description
Stannic chloride pentahydrate is a white colored solid. Stannic chloride pentahydrate is soluble in water. Stannic chloride pentahydrate is toxic by ingestion, inhalation and skin absorption. Stannic chloride pentahydrate is used to make perfumes and dyes.
Air & Water Reactions
Soluble in water. Reacts with water to form Hydrochloric Acid in dense white fumes [Merck 11th ed. 1989].
Reactivity Profile
Acidic salts, such as STANNIC CHLORIDE, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.
Hazard
Toxic material
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Safety Profile
A poison by intraperitoneal and intravenous routes. Mutation data reported. A corrosive liquid. When heated to decomposition it emits toxic vapors of tin and Cl-.
Potential Exposure
Hydrated stannic chloride is used for fixing certain textile dyes, and for treating silk to give weight to the fabric.
Shipping
UN2440 Stannic chloride, pentahydrate, Hazard class: 8; Labels: 8-Corrosive material.
Incompatibilities
Reacts violently with water, forming corrosive hydrochloric acid and tin oxide fumes. Reacts with turpentine, alcohols, and amines, causing fire and explosion hazard. Attacks many metals; some forms of plastics, rubber, and coatings. Reacts with moist air to form hydrochloric acid and dense white fume.
Properties of Tin(IV) chloride
| Melting point: | 56 °C |
| Density | 2.040 |
| solubility | very soluble in H2O; soluble in ethanol |
| form | lumps |
| color | Light Yellow |
| Water Solubility | soluble |
| Merck | 14,8774 |
| Stability: | Stable, but may decompose in contact with moisture or water. Incompatible with strong acids. |
| CAS DataBase Reference | 10026-06-9(CAS DataBase Reference) |
| EPA Substance Registry System | Stannane, tetrachloro-, pentahydrate (10026-06-9) |
Safety information for Tin(IV) chloride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Tin(IV) chloride
| InChIKey | KHMOASUYFVRATF-UHFFFAOYSA-J |
Tin(IV) chloride manufacturer
ARRAKIS INDUSTRIES LLP
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran
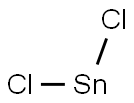

You may like
-
 10026-06-9 99%View Details
10026-06-9 99%View Details
10026-06-9 -
 STANNIC CHLORIDE 10026-06-9 99%View Details
STANNIC CHLORIDE 10026-06-9 99%View Details
10026-06-9 -
 10026-06-9 99%View Details
10026-06-9 99%View Details
10026-06-9 -
 Tin(Iv) Chloride Pentahydrate CAS 10026-06-9View Details
Tin(Iv) Chloride Pentahydrate CAS 10026-06-9View Details
10026-06-9 -
 Stannic chloride, hydrate CAS 10026-06-9View Details
Stannic chloride, hydrate CAS 10026-06-9View Details
10026-06-9 -
 STANNIC CHLORIDE (pentahydrate) CAS 10026-06-9View Details
STANNIC CHLORIDE (pentahydrate) CAS 10026-06-9View Details
10026-06-9 -
 Tin(IV) chloride pentahydrate CAS 10026-06-9View Details
Tin(IV) chloride pentahydrate CAS 10026-06-9View Details
10026-06-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
