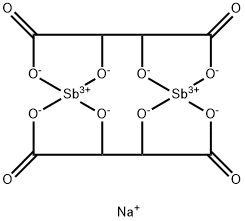
Sodium Stibogluconate
Synonym(s):Sodium Stibogluconate - CAS 16037-91-5 - Calbiochem;SSG, Antimony Sodium Gluconate, NaSbv, PTP Inhibitor XXIV, SHP1 Inhibitor IV
- CAS NO.:16037-91-5
- Empirical Formula: C12H37Na3O27Sb2
- Molecular Weight: 925.9
- MDL number: MFCD01746582
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-18 17:01:59

What is Sodium Stibogluconate?
Toxicity
The main symptoms of antimony overdosage are gastro-intestinal disturbances (nausea, vomiting and severe diarrhoea). Haemorrhagic nephritis and hepatitis may also occur.
Description
Sodium stibogluconate is a pentavalent antimony derivative that is highly active against Leishmania amastigotes in macrophages. Sodium stibogluconate has diverse effects on both this intracellular parasite and its host cell.
Description
Sodium stibogluconate (Na3Sb2C12H38O26), also known as sodium antimony(V) gluconate, is a solid ionic compound that is freely soluble in water. Its precise structure is unknown; the one shown here is idealized based on the atomic formula.
Workers in India prepared sodium stibogluconate in the late 1940s via the reaction of antimony pentoxide with sodium hydroxide. It has been used since then as an injectable drug for treating visceral leishmaniasis, a fatal insect-borne microbial disease.
Visceral leishmaniasis is the most virulent form of the disease, which is caused by protozoa of the genus Leishmania. The parasite is transported by several genera of flying insects and introduced into the skin by insect bites. The initial symptom is skin ulcers, followed by fever, low red blood count, and enlarged internal organs. It is a prevalent disease among poor, malnourished populations.
Over the years, Indian scientists observed that in areas where the drinking water is contaminated with As(III), Individuals not only suffer from arsenic toxicity, but they do not respond well to the leishmaniasis treatment. The Sb(V) in stibogluconate is reduced to Sb(III) by the Leishmania parasite before the antimony disrupts the bug’s defenses; As(III) works the same way. Researchers now believe that Leishmania mutations may have caused the microbe to become resistant to As(III) and Sb(III).
In 2005, the Indian government stopped recommending the use of antimony-based drugs, but sodium stibogluconate and others continue to be used because alternatives are significantly more expensive. As a result, cure rates plunged by ≈60% between 2006 and 2010.
The Uses of Sodium Stibogluconate
Sodium stibogluconate is a pentavalent antimony derivative that is highly active against Leishmania amastigotes in macrophages. Sodium stibogluconate has diverse effects on both this intracellular parasite and its host cell.
The Uses of Sodium Stibogluconate
Sodium Stibogluconate is an intermediate used to prepare Dihydropyrido[2,3-d]pyrimidines as a new class of antileishmanial agents.
Background
Sodium stibogluconate is a medicine used to treat leishmaniasis and is only available for administration by injection. It belongs to the class of medicines known as the pentavalent antimonials. Sodium stibogluconate is sold in the UK as Pentostam (manufactured by GlaxoSmithKline). Widespread resistance has limited the utility of sodium stibogluconate, and in many parts of the world, amphotericin or miltefosine are used instead. It is also being investigated as an anti-tumor agent.
Indications
For the treatment of various types of a protozoal infection called leishmaniasis, which may result from sandfly bites in tropical and temperate parts of the world. Also investigated for use/treatment in cancer/tumors (unspecified) and solid tumors.
What are the applications of Application
Sodium stibogluconate is a robust inhibitor of PTPases, including PTPase1 (SHP-1), SHP-2, and PTP1B
Antimicrobial activity
It has low activity against the extracellular promastigote stage of Leishmania spp. in vitro, but is active against amastigotes in macrophages. The trivalent form is considered to be the toxophore, with metabolism of SbV to toxic SbIII in the host cell macrophage and the parasite; the level of metabolism is higher in amastigotes than promastigotes. Variation in the sensitivity of different Leishmania species may contribute to differences in clinical response. It is more active against visceral than cutaneous leishmaniasis in animal models. Sodium stibogluconate cures CNS infections with T. brucei in rodents.
Acquired resistance
Unresponsiveness and relapse of L. donovani infections in
Bihar State, India (over 60% of cases), is due to increasing
acquired resistance. In laboratory-generated and clinical
isolates,
resistant Leishmania promastigotes show increased
levels of intracellular thiols, for example trypanothione, to
which SbIII is conjugated and extruded by efflux pumps.
Lack of response is also reported in patients with mucosal
leishmaniasis caused by L. braziliensis. Relapse is common
in patients with visceral leishmaniasis who are immunosuppressed,
for example by HIV infection, but this is due to
pathogen interaction and the immune dependence of drug
activity and not acquired resistance. High mortality has been
reported in treatment of these co-infection cases.
Pharmaceutical Applications
A pentavalent antimonial of uncertain chemical composition; probably a complex mixture of polymeric forms. There is batchto- batch variation and solutions may contain 32–34% pentavalent antimony (SbV). The structural formula is conjectural as studies have identified a mixture of non-complexed SbV and large polymeric gluconate complexes. Chemical composition also depends on concentration and time. It is freely soluble in water.
Pharmacokinetics
The mode of action of sodium stibogluconate is not clearly understood. In vitro exposure of amastigotes to 500 mg pentavalent antimony/ml results in a greater than 50% decrease in parasite DNA, RNA protein and purine nucleoside triphosphate levels. It has been postulated that the reduction in ATP (adenosine triphosphate) and GTP (guanosine triphosphate) synthesis contributes to decreased macromolecular synthesis.
Pharmacokinetics
Peak concentrations of about 12–15 mg antimony/L are achieved in serum 1 h after a dose of 10 mg/kg. There is a slow accumulation in the central compartment, and tissue concentrations reach a maximum after several days. In contrast to trivalent derivatives, pentavalent antimonials are not accumulated by erythrocytes, but there is evidence of protein binding. Antimony is detected in the skin for at least 5 days after treatment. Some of the dose of SbV is converted to SbIII, possibly by the liver or by macrophages. It is rapidly excreted into urine with a half-life of about 2 h; 60–80% of the dose appears in the urine within 6 h of parenteral administration. In a study on structurally related meglumine antimonate the pharmacokinetics of SbV and SbIII were similar as measured by serum and urine levels.
Clinical Use
Visceral, cutaneous and mucocutaneous leishmaniasis
The combination with paromomycin has been used in unresponsive
cases and in relapses of visceral and cutaneous
leishmaniasis.
Clinical Use
Sodium antimony gluconate (Pentostam) is a pentavalent antimonialcompound intended primarily for the treatment ofvarious forms of leishmaniasis. It is available from the CDCas the disodium salt, which is chemically stable and freelysoluble in water. The 10% aqueous solution used for eitherintramuscular or intravenous injection has a pH of approximately5.5. Like all antimonial drugs, this drug has a lowtherapeutic index, and patients undergoing therapy with itshould be monitored carefully for signs of heavy metal poisoning.Other organic antimonial compounds are used primarilyfor the treatment of schistosomiasis and other flukes.The antileishmanial action of sodium stibogluconate requiresits reduction to the trivalent form, which is believedto inhibit phosphofructokinase in the parasite.
Side Effects
The toxic effects are limited by the rapid excretion, but cumulative toxicity increases in proportion to dose. Myalgia, arthralgia, anorexia and electrocardiographic changes have been reported with high-dose regimens. In particular, development of ventricular tachyarrhythmias associated with prolongation of the QT interval has been recorded. Hepatocellular damage, hepatic and renal functional impairment and pancreatitis have also been reported. The changes are reversible on discontinuation of treatment.
Safety Profile
Poison by intraperitoneal route. When heated to decomposition it emits toxic fumes of Sb.
Veterinary Drugs and Treatments
Sodium stibogluconate is used for the treatment of leishmaniasis in dogs.
Drug interactions
otentially hazardous interactions with other drugs
P
Antifungals: possible increased risk of arrhythmias
with amphotericin - give 14 days apart.
Metabolism
Not Available
Metabolism
No data on possible metabolic pathways.
Elimination occurs in two phases; a rapid initial phase, in
which the majority of a dose is excreted via the kidneys
within 12 hours, and a slower phase, possibly reflecting
reduction to trivalent antimony.
Properties of Sodium Stibogluconate
| Melting point: | >250°C (dec,) |
| storage temp. | 2-8°C |
| solubility | H2O: 1 mg/mL at ~75 °C |
| form | solid |
| color | pale yellow |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 16037-91-5 |
Safety information for Sodium Stibogluconate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for Sodium Stibogluconate
| InChIKey | VJCZMGLJTJZFEN-ZYPIZMQFSA-K |
Sodium Stibogluconate manufacturer
ShanPar Industries Pvt Ltd
DeFINE CHEMICALS
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 16037-91-5 Sodium stibogluconate 99%View Details
16037-91-5 Sodium stibogluconate 99%View Details
16037-91-5 -
 16037-91-5 98%View Details
16037-91-5 98%View Details
16037-91-5 -
 Sodium stibogluconate 98%View Details
Sodium stibogluconate 98%View Details
16037-91-5 -
 Sodium Stibogluconate CAS 16037-91-5View Details
Sodium Stibogluconate CAS 16037-91-5View Details
16037-91-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1