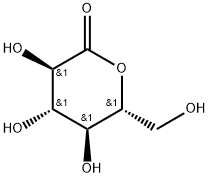
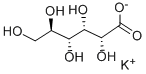
Sodium gluconate
Synonym(s):Sodium gluconate;D -Gluconate sodium salt;D -Gluconic acid sodium salt;2,3,4,5,6-Pentahydroxycaproic acid sodium salt;Gluconic acid sodium salt
- CAS NO.:527-07-1
- Empirical Formula: C6H13NaO7
- Molecular Weight: 220.15
- MDL number: MFCD00064210
- EINECS: 208-407-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 12:07:08
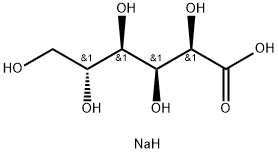
What is Sodium gluconate ?
Description
Sodium gluconate is the organic sodium salt of gluconic acid. Sodium gluconate is a chelator that forms stable complexes with various ions and ultimately prevents these ions from engaging in chemical reactions. Gluconates are naturally occurring substances that freely dissociate to the gluconate anion and its respective cations. Being fully biodegradable and non-toxic, it represents an environment friendly alternative to the common chelating agents used in cosmetics such as EDTA. In addition to this, sodium gluconate has a low acute toxicity to aquatic organisms.
Chemical properties
Sodium gluconate is a white to tan, granular to fine, practically odourless crystalline powder. It is very soluble in water, sparingly soluble in alcohol and insoluble in ether.
The Uses of Sodium gluconate
Sodium Gluconate is an excellent chelating agent, water reducer and efficient plasticizer. It is non-toxic, non corrosive and easily biodegradable (98% after 2 days) grade. Aqueous solutions of sodium gluconate are resistant to oxidation and reduction, even at high temperatures. Sodium Gluconate is recommended for inks, paints and coatings.
Definition
ChEBI: Sodium gluconate is an organic sodium salt having D-gluconate as the counterion. It has a role as a chelator. It contains a D-gluconate.
Synthesis
The calcium gluconate is added into the reaction kettle. Add sulfuric acid aqueous solution while stirring. After mixing for one hour, let it stand for a while and then get it filtered. The filter residue is CaSO4 and gets it removed. The filtrate is added into the neutralization kettle, and a proper amount of Na2CO3 aqueous solution is added to neutralize it. Finally get sodium gluconate through concentration, filtration and drying.
Regulations
In Europe, sodium gluconate is listed as a generally permitted food additive (E576) and may be added to all foodstuffs, following the "quantum satis" principle, as long as no special regulation restricts the use.
The US Food and Drug Administration (FDA) assigned sodium gluconate the “generally recognised as safe” (GRAS) status and permitted its use in food without limitation other than current good manufacturing practice.
Sodium gluconate is exempted from the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) by means of Commission Regulation (EC) No. 987/2008 of 8 October, 2008 (amending Annex IV). As a consequence thereof, there is no need to register sodium gluconate.
Properties of Sodium gluconate
| Melting point: | 170-175 °C |
| alpha | [α]D20 +11~+13° (c=10, H2O) |
| storage temp. | Store below +30°C. |
| solubility | H2O: 0.1 g/mL, clear |
| form | Crystalline Powder |
| color | White to light beige |
| PH | 7.0-8.0 (100g/l, H2O, 20℃) |
| Odor | wh. to ylsh. cryst. powd., pleasant odor |
| Water Solubility | Very soluble in water; sparingly soluble in alcohol; insoluble in ether. |
| Merck | 14,4456 |
| BRN | 3919651 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 527-07-1(CAS DataBase Reference) |
| EPA Substance Registry System | Sodium gluconate (527-07-1) |
Safety information for Sodium gluconate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Sodium gluconate
Sodium gluconate manufacturer
JSK Chemicals
Vora Brothers
Sona Enterprise
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Sodium Gluconate 98%View Details
Sodium Gluconate 98%View Details -
 SODIUM GLUCONATE 99%View Details
SODIUM GLUCONATE 99%View Details -
 Sodium gluconate 98%View Details
Sodium gluconate 98%View Details -
 Sodium Gluconate CAS 527-07-1View Details
Sodium Gluconate CAS 527-07-1View Details
527-07-1 -
 Sodium Gluconate CASView Details
Sodium Gluconate CASView Details -
 Sodium Gluconate USP/FCCView Details
Sodium Gluconate USP/FCCView Details
527-07-1 -
 Sodium Gluconate 99, Powder, Grade: IndustrialView Details
Sodium Gluconate 99, Powder, Grade: IndustrialView Details
527-07-1 -
 Pharma grade Powder sodium Gluconate, Packaging Size: 50kg, Packaging Type: Paper DrumView Details
Pharma grade Powder sodium Gluconate, Packaging Size: 50kg, Packaging Type: Paper DrumView Details
100817-46-7
