Sodium bisulfite
Synonym(s):Sodium bisulfite, Sodium bisulfite solution, Bisulfite;Sodium disulfite;Sodium hydrogen sulfite;Sodium hydrogensulfite;Sodium metabisulfite, Sodium pyrosulfite, Disulfite, Pyrosulfite
- CAS NO.:7631-90-5
- Empirical Formula: HNaO3S
- Molecular Weight: 104.06
- MDL number: MFCD00003530
- EINECS: 231-548-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:53
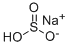
What is Sodium bisulfite?
Description
Sodium bisulfite (NaHSO3) is a weakly acidic compound that is usually used as a mild reducing agent. Sodium bisulfite is typically used as an aqueous solution. A common application is in the workup of reactions involving bromine or iodine.
Chemical properties
white powder
Chemical properties
Sodium bisulfite is a white crystalline solid. Slight odor of sulfur dioxide and a disagreeable taste. Slowly oxidized to the sulfate on exposure to air
The Uses of Sodium bisulfite
sodium bisulfite is a preservative and anti-oxidant, it is most frequently used as a pH adjuster.
The Uses of Sodium bisulfite

To the SM (1.77 Kg, 8.58 mol) in anhydrous DMF (8 L) under N2 at 10 C was added NIS (1.93 Kg, 8.58 mol) in portions over 10 min. The reaction mixture was stirred at RT for 2 h, after which time it was cooled using an ice bath and quenched with sat aq Na2CO3 (5 L) and extracted with EtOAc (2 x 15 L). The combined organics were washed with sat aq Na2CO3 (2 x 5 L), H2O (3 x 2 L), dried (Na2SO4), and concentrated in vacuo. The resulting crude material was purified by silica gel column chromatography (25-40% EtOAc/hexanes) to provide the product. [1.47 Kg, 57% over 2 steps]
The Uses of Sodium bisulfite
Reducing agent; convenient source of sulfur dioxide.
The Uses of Sodium bisulfite
As disinfectant and bleach, particularly for wool; in dyeing for preparing hot and cold indigo vats; in paper-making in place of sodium hyposulfite to remove Cl from bleached fibers; as stripper (reducer) in laundering; to remove permanganate stains from skin and clothing; to render certain dyes sol; manufacture of sodium hydrosulfite; coagulating rubber latex; as preservative for deteriorative liqs or solutions used for technical purposes; as antiseptic in fermentation industries. As preservative and bleach in food. Pharmaceutic aid (antioxidant).
The Uses of Sodium bisulfite
Sodium Bisulfite is a preservative that exists as a powder, with a solubility of 1 g in 4 ml of water. it prevents discoloration and inhib- its bacterial growth. it is used in dried fruit to inhibit browning and maintain the bright color. it is found in reconstituted lemon juice. see sulfur dioxide.
What are the applications of Application
Sodium bisulfite, mixture of NaHSO3 and Na2S2O5 is an antioxidant and antimicrobial agent for DNA methylation studies
Background
Sodium bisulfite has been used externally for parasitic skin diseases and as a gastrointestinal antiseptic.
Definition
ChEBI: Sodium hydrogensulfite is an inorganic sodium salt having hydrogensulfite as the counterion. It has a role as a mutagen and an allergen. It is an inorganic sodium salt and a sulfite salt. It contains a hydrogensulfite.
General Description
Also known as sodium acid sulfite or sodium hydrogen sulfite, NaHS03 is white crystals or crystalline powder that has a slight sulfurous odor and taste.Specific gravity 1.48. Strong irritant to skin and tissue. It is soluble in water,insoluble in alcohol,unstable in air,and non-combustible. Derived by saturating sodium carbonate solution with sulfur dioxide and the solution crystallized. Used as a chemical(sodium salts, cream of tartar), in vat dye preparation, textiles (antichlor, mordant, discharge), food preservative, bleaching groundwood, wool,etc., reducing agent, fermentation, antiseptic, cask sterilization (brewing), copper and brass plating,color preservative for pale crepe rubber, wood pulp digestion, analytical reagent,and as a dietary supplement.
Air & Water Reactions
Water soluble.
Reactivity Profile
Sodium bisulfite is a reducing agent. Emits highly toxic gaseous sulfur dioxide gas when heated to decomposition or on contact with mineral acids [Sax, 9th ed., 1996, p. 2949].
Hazard
Not permitted in meats and other sources of vitamin B 1 , strong irritant to skin and tissue.
Health Hazard
Powder is irritating to eyes, nose, and throat and can irritate skin. Ingestion may cause irritation of stomach. Very large doses cause violent colic, diarrhea, depression, and death.
Fire Hazard
Flammable/combustible material. May ignite on contact with moist air or moisture. May burn rapidly with flare-burning effect. Some react vigorously or explosively on contact with water. Some may decompose explosively when heated or involved in a fire. May re-ignite after fire is extinguished. Runoff may create fire or explosion hazard. Containers may explode when heated.
Contact allergens
Sodium bisulfite is mainly used as an antioxidant in pharmaceutical products, as a disinfectant or bleach, and in the dye industry. The bisulfite of commerce consists chiefly of metabisulfite and possesses the same properties as the true bisulfite. So, the allergen to be tested in products containing disulfite is the corresponding metabisulfite.
Safety Profile
Poison by intravenous and intraperitoneal routes. Moderately toxic by ingestion. A corrosive irritant to skin, eyes, and mucous membranes. Mutation data reported. An allergen. When heated to decomposition it emits toxic fumes of SO, and Na2O. See also SULFUROUS ACID and SULFITES
Potential Exposure
Sodium bisulfite is used in the digestion of wood pulp, in the tanning of leather; in the dyeing of textiles; as a photographic reducing agent; as a food preservative; as an additive in electroplating; as disinfectant, bleach, antioxidant, and as inhibitor of yeast and bacteria in winemaking.
Metabolism
Not Available
Shipping
UN2693 Bisulfites, inorganic, aqueous solutions, n.o.s., Hazard class: 8; Labels: 8-Corrosive material. UN3260 Bisulfites, inorganic, solid, n.o.s., Hazard class: 8; Labels: 8-Corrosive material.
Purification Methods
Crystallise it from hot H2O (1mL/g). Dry it at 100o under vacuum for 4hours.
Incompatibilities
Aqueous solution is a weak acid. Incompatible with strong mineral acids forming a toxic sulfur dioxide gas. A strong reducing agent, sodium bisulfite is incompatible with oxidizers, such as perchlorates, peroxides, permanganates, chlorates, and nitrates. Reacts with bases forming sulfate. Slowly oxidizes to sulfate in air. Heat causes decomposition. Slowly oxidized to the sulfate on exposure to air. Contact with oxidizers or acids forms sulfur dioxide gas. Attacks some metals in the presence of moisture.
Waste Disposal
Dump into water, add soda ash, then neutralize with HCl; flush to sewer with large volumes of water.
Properties of Sodium bisulfite
| Melting point: | 150 °C |
| Density | 1.48 |
| storage temp. | Store at RT. |
| solubility | 300 g/L |
| form | Powder/Solid |
| appearance | White solid |
| Specific Gravity | 1.48 |
| color | White |
| Odor | Slight odor of sulfur dioxide |
| Water Solubility | 300 g/L |
| Merck | 13,8660 |
| Stability: | Stable. Incompatible with strong oxidizing agents, strong acids. |
| CAS DataBase Reference | 7631-90-5(CAS DataBase Reference) |
| EPA Substance Registry System | Sodium bisulfite (7631-90-5) |
Safety information for Sodium bisulfite
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for Sodium bisulfite
| InChIKey | DWAQJAXMDSEUJJ-UHFFFAOYSA-M |
Sodium bisulfite manufacturer
C.H. Chemicals
ASM Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 SODIUM BI-SULPHITE 99%View Details
SODIUM BI-SULPHITE 99%View Details -
 SODIUM META BI-SULPHITE 99%View Details
SODIUM META BI-SULPHITE 99%View Details -
 Sodium meta bi sulphate 99%View Details
Sodium meta bi sulphate 99%View Details -
 Sodium Meta Bi Sulphate 99%View Details
Sodium Meta Bi Sulphate 99%View Details -
 Sodium hydrogen sulfite 99%View Details
Sodium hydrogen sulfite 99%View Details -
 Sodium Meta Bi Sulphate CASView Details
Sodium Meta Bi Sulphate CASView Details -
 Sodium Meta Bi Sulphate CASView Details
Sodium Meta Bi Sulphate CASView Details -
 sodium meta bi- sulphite CASView Details
sodium meta bi- sulphite CASView Details
