SODIUM NITROPRUSSIDE
Synonym(s):Nitroprusside sodium;SNP;Sodium nitroferricyanide(III) dihydrate;Sodium nitroprusside;Sodium pentacyanonitrosylferrate
- CAS NO.:14402-89-2
- Empirical Formula: C5FeN6NaO-
- Molecular Weight: 238.93
- MDL number: MFCD00003506
- EINECS: 238-373-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-03 09:04:23
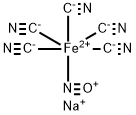
What is SODIUM NITROPRUSSIDE?
Description
Sodium nitroprusside is a powerful, instantaneous-acting intravenous drug used to lower blood pressure in hypertensive crises. The hypotensive effect is caused by peripheral vasodilation resulting from a direct effect on both arterial and venous vessels.
The Uses of SODIUM NITROPRUSSIDE
Sodium Nitroprusside is a potent vasodilator working through releasing NO spontaneously in blood
Definition
ChEBI: An organic sodium salt that is the disodium salt of nitroprusside.
brand name
Nipride (Roche); Nitropress (Abbott); Nitropress (Hospira).
Biological Functions
Sodium nitroprusside (Nipride) is a potent directly acting vasodilator capable of reducing blood pressure in all patients, regardless of the cause of hypertension. It is used only by the intravenous route for the treatment of hypertensive emergencies. The pharmacological activity is caused by the nitroso moiety. The actions of the drug are similar to those of the nitrites and nitrates that are used as antianginal agents. The action of the nitrovasodilators depends on the intracellular production of cGMP.
General Description
Sodium nitroprusside,sodium nitroferricyanide, disodium pentacyanonitrosylferrate(2) Na2[Fe(CN)5NO] (Nipride, Nitropress), is one of themost potent blood pressure–lowering drugs. Its use is limitedto hypertensive emergencies because of its short durationof action. The effectiveness of sodium nitroprusside asan antihypertensive has been known since 1928, but notuntil 1955 was its efficacy as a drug established. The drugdiffers from other vasodilators, in that vasodilation occurs inboth venous and arterial vascular beds. Sodium nitroprussideis a reddish brown water-soluble powder that is decomposedby light when in solution. The hypotensive effect ofthe chemical is a result of the formation of NO in situ (discussedunder the heading, “Nitrovasodilators”), elevatingcellular levels of cGMP. Sodium nitroprusside is metabolizedby the liver, yielding thiocyanate. Because thiocyanateis excreted by the kidneys, patients with impaired renalfunction may suffer thiocyanate toxicity.
Mechanism of action
Sodium nitroprusside is not an active hypotensive drug until metabolized to its active metabolite, NO, the mechanism of
action of which has been previously described. Studies with sodium nitroprusside suggest that it releases
NO by its interaction with glutathione or with sulfhydryl groups in the erythrocytes and tissues to form a S-nitrosothiol
intermediate, which spontaneously produces NO, which in turn freely diffuses into the VSM, thereby increasing
intracellular cGMP concentration. NO also activates K+
channels, which leads to hyperpolarization and relaxation.
The hypotensive effect of sodium nitroprusside is augmented by concomitant use of other hypotensive agents and is
not blocked by adrenergic blocking agents. It has no direct effect on the myocardium, but it may exert a direct coronary
vasodilator effect on VSM. When sodium nitroprusside is administered to hypertensive patients, a slight increase in
heart rate commonly occurs, and cardiac output usually is decreased slightly. Moderate doses of sodium nitroprusside
in patients with hypertension produce renal vasodilation without an appreciable increase in renal blood flow or
decrease in glomerular filtration.
Intravenous infusion of sodium nitroprusside produces an almost immediate reduction in blood pressure. Blood
pressure begins to rise immediately when the infusion is slowed or stopped and returns to pretreatment levels within 1
to 10 minutes.
Pharmacology
In contrast to hydralazine, minoxidil, and diazoxide, sodium nitroprusside relaxes venules as well as arterioles. Thus, it decreases both peripheral vascular resistance and venous return to the heart. This action limits the increase in cardiac output that normally follows vasodilator therapy. Sodium nitroprusside does not inhibit sympathetic reflexes, so heart rate may increase following its administration even though cardiac output is not increased. Renal blood flow remains largely unaffected by sodium nitroprusside, because the decrease in renal vascular resistance is proportional to the decrease in mean arterial pressure. As with all vasodilators, plasma renin activity increases.
Pharmacokinetics
Sodium nitroprusside undergoes a redox reaction that releases cyanide. The cyanide that is produced is rapidly converted into thiocyanate in the liver by the enzyme thiosulfate sulfotransferase (rhodanase) and is excreted in the urine. The rate-limiting step in the conversion of cyanide to thiocyanate is the availability of sulfur donors, especially thiosulfate. Toxic symptoms of thiocyanate begin to appear at plasma thiocyanate concentrations of 50 to 100 mg/mL. The elimination half-life of thiocyanate is 2.7 to 7.0 days when renal function is normal but longer in patients with impaired renal function.
Clinical Use
Sodium nitroprusside is used in the management of hypertensive crisis. Although it is effective in every form of hypertension because of its relatively favorable effect on cardiac performance, sodium nitroprusside has special importance in the treatment of severe hypertension with acute myocardial infarction or left ventricular failure. Because the drug reduces preload (by venodilation) and afterload (by arteriolar dilation), it improves ventricular performance and in fact is sometimes used in patients with refractory heart failure, even in the absence of hypertension.
Side Effects
The most commonly encountered side effects of sodium
nitroprusside administration are nausea, vomiting, and
headache, which quickly dissipate when the infusion is
terminated. When sodium nitroprusside treatment extends
for several days, there is some danger of toxicity
owing to the accumulation of its thiocyanate metabolite.
Thiocyanate intoxication includes signs of delirium
and psychosis; hypothyroidism also may occur. If nitroprusside
is administered for several days, thiocyanate
levels should be monitored.
Close supervision is required when nitroprusside is
used because of the drug’s potency and short duration
of action.
Safety Profile
Human poison by inhalation and intravenous routes. Experimental poison by ingestion, intraperitoneal, and intravenous routes. Human systemic effects: increased intracranial pressure, general anesthesia, change in heart rate, and metabolic acidosis. An experimental teratogen. Used as a vasodilator for short-term treatment of severe hypertension. Mixtures with sodium nitrite explode when heated. When heated to decomposition it emits toxic fumes of NOx, CN-, and Na2O.
Synthesis
It is synthesized by successive reactions including the reaction of potassium ferrocyanide with nitric acid, which forms potassium nitroprusside (22.6.5), which is further transformed to copper nitroprusside (22.6.6), and reaction of this with sodium carbonate gives sodium nitroprusside (22.6.7).
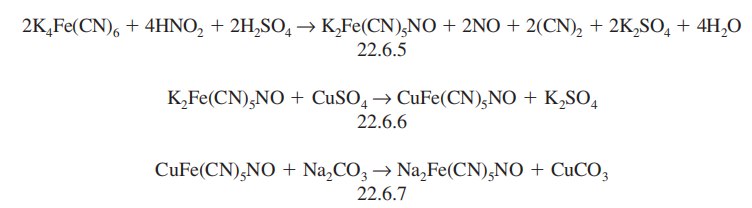
Drug interactions
Potentially hazardous interactions with other drugs
Anaesthetics: enhanced hypotensive effect.
Metabolism
The onset of the hypotensive action of sodium nitroprusside
is rapid, within 30 seconds after intravenous
administration. If a single dose is given, the action lasts
for only a couple of minutes. Therefore, sodium nitroprusside
must be administered by continuous intravenous
infusion. After the infusion is stopped, blood
pressure returns to predrug levels within 2 to 3 minutes.
Nitroprusside is metabolically degraded by the liver,
yielding thiocyanate. Because thiocyanate is excreted
by the kidney, toxicities due to this compound are most
likely in patients with impaired renal function.
Properties of SODIUM NITROPRUSSIDE
| Density | 1.72 |
| storage temp. | 4°C, protect from light |
| solubility | ≥ 11.2mg/mL in DMSO |
| form | Liquid |
| color | Clear tan-gold |
| EPA Substance Registry System | Ferrate(2-), pentakis(cyano-.kappa.C)nitrosyl-, disodium, (OC-6-22)- (14402-89-2) |
Safety information for SODIUM NITROPRUSSIDE
Computed Descriptors for SODIUM NITROPRUSSIDE
SODIUM NITROPRUSSIDE manufacturer
Atulya Chemicals
Zama Chemical
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 Sodium Nitroprusside Cas no:13755-38-9, For Industrial, Packaging Type: BagView Details
Sodium Nitroprusside Cas no:13755-38-9, For Industrial, Packaging Type: BagView Details
13755-38-9 -
 Sodium Nitroprusside Crystal LAB GREADView Details
Sodium Nitroprusside Crystal LAB GREADView Details
13755-38-9 -
 Sodium Nitroprusside, PrescriptionView Details
Sodium Nitroprusside, PrescriptionView Details
14402-89-2 -
 Sodium Nitroprusside Powder, IPView Details
Sodium Nitroprusside Powder, IPView Details
13755-38-9 -
 Sodium NitroprussideView Details
Sodium NitroprussideView Details
13755-38-9 -
 Sodium Nitroprusside PowderView Details
Sodium Nitroprusside PowderView Details
13755-38-9 -
 Sodium Nitroprusside Crystal PowderView Details
Sodium Nitroprusside Crystal PowderView Details
13755-38-9 -
 Sodium NitroprussideView Details
Sodium NitroprussideView Details
13755-38-9
